A ledek napjainkban egyre elterjedtebbek.Ilyenek vannak szinte minden készülékben az öngyújtóktól kezdve az űrhajókig. A fénykibocsátó dióda vagy LED neve az angol Light Emitting Diode rövidítéséből származik. A dióda által kibocsátott fény színe a félvezető anyag összetételétől, ötvözőitől függ. A LED jellemzően egyszínű inkoherens keskeny spektrumú fényt bocsát ki. A fény spektruma az infravöröstől az ultraibolyáig terjedhet, de hozzáférhetőek már hideg meleg és természetes fehér fényű LED-ek is.
A fénykibocsátás úgy keletkezik, hogy a diódára adott áramforrás a dióda anyagában levő atomok elektronjait gerjeszti, amitől azok nagyobb energiaszintű elektronpályára lépnek, majd ezek miközben visszatérnek eredeti helyükre, fotonokat bocsátanak ki. Nyitóirányú áram esetén a PN átmeneten az elektronok a N rétegből a P-be, a lyukak a P rétegből az N-be diffundálnak. A diffúziós kisebbségi és többségi töltéshordozók között rekombinációs folyamat indul meg, melynek során a felszabaduló energia fotonok formájában kisugárzódik. Nagyobb feszültség hatására nagyobb a kisugárzott fotonok mennyisége, egészen egy bizonyos nyitóirányú áramértékig, ahonnan már nem számottevő a változás.
A LED előnye, hogy a kimeneti fény előállításához alacsony áramot és feszültséget igényel, így alacsony az üzemeltetési költsége. Nagy a kapcsolási sebessége, kis helyen elfér, ütésálló és hosszú az élettartama. A hirtelen kiégés helyett lassan használódik el. A kis villamos teljesítményből fakadóan hőtermelése kicsi, így a fényforrás, és a megvilágított felület távolsága minimalizálható, jelentősen csökkentve a fényveszteséget. Hátránya az ára, de a gyártási technológiák fejlődésével és azok elterjedésével várhatóan jelentősen mérséklődik rövid időn belül. (Jelenleg egy 300W-os lámpát egy hasonló teljesítményű HPS árának duplájáért lehet megépíteni és nagyjából háromszorosáért megvenni készen).
Felfedezése
1900-as évek elején Henry Round, a Marconi Labs szakembere fedezte fel az Elektroluminancia jelenségét.
1920-as év közepén az orosz Oleg Vladimirovich Lossew független kutató készítette el az első LED-et. Kutatásai eredményét közölték német, orosz és angol szaklapokban, de munkásságát az akkori tudományos világ nem ismerte fel, mivel a kibocsájtott fény intenzitása igen csekély volt.
1955-ben Rubin Braunstein, az amerikai rádiózási vállaltnál készítette el az első kisérleti infravörös tartományban sugárzó LED diódát, aminek az alapanyaga gallium arzenid (GaAs) volt.
1961 ben az amerikai Texas Instruments vállaltnál, Bob Biard és Gary Pittman, tovább kísérletezett, gallium arzenit alapú infravörös (láthatatlan) tartományban fényt sugárzó diódákkal. Biard és Pittman felismerték munkájuk fontosságát, és szabadalmaztatták az infravörös LED-et.
1962 ben Nick Holonyak Jr. a General Electric Company szakembere kifejlesztette az első, látható tartományban sugárzó LED-et.
1972-ben. Holonyak korábbi tanítványa Dr. M. George Craford megalkotta az első gyakorlatban is használható sárga LED-et. Nem sokkal később a sárgánál 10 x fényesebb vörös és vörös-narancs LED-eket is piacra dobták.
1971 ben jelentett Nagy áttörést a kék LED felfedezése, amely első verzióban Jacques Pankove az RCA Laboratories szakembere nevéhez fűződik.
1993-ban A kék LED megdöbbentően nagy fényerő növekedést ért el Shuji Nakamura a Nichia Corporation kutatója munkásságának köszönhetően. Ettől kezdve napjainkig több nagyvállalat tökéletesített technikával gyárt LED chipeket, de az alapelvek 1993 óta nem változtak.
1999-ben a Philips Lumileds legyártotta az első folyamatos üzemű 1 wattos LED-et. Ez a LED mérete szerint sokkal nagyobb lett, mint hagyományos társai. Ezek a LED-ek már csak hűtőbordára szerelve használhatóak, és ezzel kezdetét vette a LED-ek világítási célú felhasználása.
LEDek változatai
A ledekről annyit mindenképpen tudni érdemes, hogy félvezető diódák amiknek egy csoportja fény leadására képes. Az általunk alkalmazni kívánt ledek a millió féle kereskedelemben kapható ledek közül viszonylag nagy teljesítménnyel és fényleadással bírnak. Fényük színtől, típustól és gyártótól függően rendkívül eltérő, 10-120lm/W között mozog, eléggé el nem ítélhető módon a kék és piros ledek 10-60 lumen/W adnak le. Általánosan 100-130 fok között mozog a sugárzási szögük, de léteznek gyárilag fókuszált 15-45-90 fokos HPL-ek is a kis teljesítményű(1-5W) fajtákból. Ezt a sugárzási szöget előtétoptikákkal lehet szűkíteni igény esetén, azonban még így is csak 85% körül ad le eredeti fénymennyiségéből.
Pár ével ezelőtt jelentek meg a piacon az ázsiai fejlesztőknél a színes nagy teljesítményű SMD ledek, melyek 10,20,30,50,100W teljesítményűek és fényintenzitásuk valamit javult, egy 100w-os piros led 6500lm leadására képes tehát 65lm/W ami egy öreg nátriumlámpa fényintenzitásának fele. Ledjeink szerelésük szempontjából sokfélék lehetnek, lyukszereltek és SMD-k. Lyukszereltnek azokat a 3, 5, 8 és 10mm átmérőjű ledeket nevezzük, melyek az elektromos készülékek visszajelző lámpáit adják általában. Ezeknek az a tulajdonságuk, hogy 20mA körüli áramot vesznek fel és a leadott fényük 1lm alatt marad, de inkább mikro-kandelben (mc) adják meg az értéket. Természetesen, ezek közt is vannak rendkívül erős fókuszált fényűek is.
LEDek szerelése és meghajtása
Az nagy teljesítményű és fényű SMD ledek általában gyári vagy tervezett foglalatba rögzíthetőek, ezek magasabb nyitófeszültséggel és hajtóárammal jellemezhetőek, kialakult szabvány nincs rájuk, ezért teljesen eltérő formájúakkal is találkozhatunk. Nyitófeszültségük 3-5W-os kategóriában 2,1-4V-ig terjed, meghajtó áramuk c.a. 350 és 1500mA között mozog. Nagyobb teljesitményu (5-100W) LED fenyforrások általában matrixban szervezett 1-3W LEDekből tevődnek össze és a táplálásukhoz 12, 35V vagy annál magasabb feszültségű árramforrás szükságes. További fontos tulajdonságuk, hogy diódalapjukon az üzem közbeni hőmérséklet 125°C fölé is tud emelkedni ezzel “megsütve” magukat, ezért ezeket a LEDeket hűtőfelületre szereljük. Számos nagyteljesítményű LEDet gyárilag csillag alakú alluminium hűtőfelületre szerelnek, egyrészt a könnyebb csavaros rögzíthetősége miatt, másrészt a lemez egy alapvető hűtést biztosít a diódának (ez nem jelenti azt, hogy nincs szükség további hűtésre). A korai LEDhalál egyik oka a túlmelegedés. Az igazán nagy teljesítményű ledek hűtését minden esetben méretezni és ellenőrizni szükséges.
Az első és legfontosabb tudnivaló a LEDek táplálásáról az, hogy a ledek elsősorban egyenárammal működnek ami azt jelenti, hogy a hálózati áramot először egyenárammá alakító tápra lesz szükségünk. Léteznek hálózati váltóáramról direkt működő ledek is, azonban jelenleg összehasonlíthatatlanul több egyenáramú led van a piacon. Ledek uzemeltetésé, emellet többféle módon is végezhetjük.
Gyári led tápok, melyek a hálózati váltóáramot egyenárammá alakítják és egy beépített kapcsolóüzemű áramgenerátorral a ledek meghajtó áramát adják. Léteznek szabályzható, kapcsolható vagy fix kivitelben. Elterjedtek a 350, 700 illetve 1000mA-es ledtápok, de léteznek ennél nagyobb áramerősséget leadni képes források is. Erősen korlátozott számú ledet tudunk használni róluk, viszont nagy előnyük az, hogy a ledek sorba forrasztásán kívül semmilyen többletmunkára vagy számításra nincs szükség a beüzemeléshez. A feszültséget szabályozva képesek automatikusan a rákapcsolt ledekhez szükséges nyitófeszültséget előállitani. Áruk 3000Ft-tól indul.
Led vezérlő panel használata akkor célszerű, ha már rendelkezünk egy szabályzott egyenfeszültséget leadni képes táppal. A panel feladata ezen feszültség további szabályozása a ledek nyitófeszültsége szerint, valamint a meghajtó áram biztosítása. Általában le- és fel-lépteti is tudják a tápfeszültséget és közel semmit nem foglalnak le a tápfeszültségből. A ráköthető minimális és maximális ledek számát, valamint a ledek típusait altalában megadják a specifikációban/használati utmutatóban. A LEDtápokhoz hasonlóan itt csak szerelési munkát kell végeznünk. Áruk 2000Ft-tól felfelé, otthoni előállítási költség 1000Ft alatt.
Általános áramgenerátorok. Ahhoz, hogy a ledünk ne menjen tönkre egyenáramra való kötés után fél percen belül, szükségünk lesz még egy áramerősséget szigorúan egyenletes szinten tartó áramgenerátorra is, ugyanis még a szabályzott feszültségű tápok is változtatják minimális mértékben leadott feszültségüket ami nagymértékű áramerősség-változással jár. Ledjeinkre ez végzetes hatással lehet.
Különböző egyszerű áramgenerátorok léteznek melyek közül a legegyszerűbb az LM317 IC. Működésének lényege, hogy két kimenő(out) lába(pin) közt 1,25V feszültségkülönbséget tart fenn, ezt kihasználva egy ellenállást beépítve a két láb közé állandó áramerősséget kapunk az ezzel sorba kötött körön belül mindenhol. Az LM317 40V feszültségig és 1,5A áramerősségig használható, azonban alacsony tápfeszültségnél nem előnyös használata ugyanis 3V feszültséget elnyel tápfeszültségünkből. Ha a tápfeszültséget nem használjuk ki teljesen akkor a fennmaradó feszültséget elfűti igen komoly hőmérséklet emelkedést okozva környezetében (előállítási költsége 200Ft alatt marad).
Egy másik rendkívül egyszerű áramgenerátor az NFET+NPN+2R. Ez mindösszesen 4db alkatrészt tartalmaz és nagy előnye az előzővel szemben, hogy magasabb feszültség és áramhasználatot enged: 60V és 20A-ig lehet használni, bízom benne senki nem próbálja meg a korlátait feszegetni. Feszültségigénye mindössze 0,5V, ami közkedvelté teszi az alacsony feszültségű alkalmazások kürében (24V alatt, összeszerelési költsége 300Ft alatt marad).
Mindkét áramgenerátorra érvényes az, hogy az IC-k elégtelen hűtése hőmegfutáshoz vezethet ami a ledjeinket ipari hulladékká minősíti. Ezt elkerülendő az IC-ket minden esetben saját hűtőbordával érdemes ellátni, ami 100Ft plusz költséget jelent a csalági kasszának darabonként.
(folyt.köv.)
Körök és alkatrészek méretezése
5mm-ES LED ÁRAMKÖR MÉRETEZÉSE
A LED-ek diódák, tehát csak az egyik irányban engedik át az áramot, csak egy polaritású feszültség hatására világítanak. Ez magyarul annyit tesz, hogy félvezetők és nem mindegy merre van a led anódja(+) és katódja(-). Ha felcserélve próbálod beszerelni nem fog világítani.
A ledek színüktől függően különböző nyitófeszültségűek, a piros, sárga és zöld 5mm-es ledek 1,8-2,2V közötti esést produkálnak, a kék és fehér ledek 3,2-4V közöttiek. Áramfelvételük 10 és 20mA közöttinek kell lenniük.
Példaként vegyünk egy piros és kék ledet, valamint egy laposelemet 4,5V-al. Ha a megfelelő lábat illesztjük az elem lábaihoz azt látjuk, hogy intenzíven világítanak. Ilyet többé ne tegyünk, igen hamar tönkre tesszük ezzel ledjeinket. Ahhoz, hogy tartósan tudjuk használni őket kicsit számolnunk kell, ugyanis a többletfeszültség és feszültségingadozás károsan hat a ledekre. A feladatunk az lenne, hogy a laposelem két pólusa közötti 4,5V feszültségkülönbséget megtarthassuk, vagyis a 4,5V feszültséget nullára csökkentsük. Elő a kék leddel, ha az előbb még nem halt meg! Számoljunk.
A kék leden 3,6V feszültség esik a 4,5-ből a led nyitásakor.
A kettő különbsége 0,9V amit egy többlet előtétellenállással arra hasznosítunk, hogy a körben folyó áram értékét beállítsuk. Hogyan? Ohm törvényével.
U=RxI
U:feszültség(V)
R:ellenállás(O,Ohm)
I:áramerősség(A,mA amper, miliamper ami az amper ezred része)
- (4,5V-3,6V)=Rx0,02A
- R=45ohm
Ilyen ellenállás nem szabványos méretű, ezért az ettől egyel nagyobb szabvány ellenállást fogjuk használni, ami a 47 ohm-os. A 47 ohmos ellenállás más áramerősséget produkál a ledben.
- 0,9V=47ohm x I
- I=0,0191A
Ezt az eltérést szemmel nem lehet látni, azonban a további számításhoz még kell, ugyanis a boltban fel fogják tenni a kérdést:”Hány vattos legyen a 47 ohm-os ellenállás?”
P:teljesítmény(W,Watt)
P=UxI
- P=0,9V x 0,0191A=0,018W A kérdésre a válasz: 1/4W-os. A biztonság kedvéért 1/2W-os jobb választás.
Vigyük haza ellenállásunkat, és építsük a led elé sorba kötve. Az ellenállásoknak nincs jelölt pólusuk, úgy kötöd őket ahogy sikerül.
Szépen világít kék fényünk, azonban mi egy pirosat is szeretnénk.
Ez ebben a körben már nem kaphat helyet hiszen az ellenállás csak 0,9V-ot dolgoz fel, ez pedig nem elég a piros led 1,8V-nyi nyitásához, ezért párhuzamosan fogjuk azt beépíteni a fenti ellenállás méretezést követve számára megfelelő előtétellenállással. Itt kívánom felhívni a figyelmet arra, hogy a piros led nyitófesze 4,5V/2 alatti, ezért két ledet is köthetünk sorba, így (4,5v-2×1,8V)=0,9V-ra kell méreteznünk az előtétellenállást.
Ha nem szeretnénk számológépet előhúzni itt egy link a ledek előtétellenállásának számításához soros és párhuzamos kapcsolás esetén.ledek előtétellenállása
Ha esetleg abban gondolkodik valaki, hogy ilyen 5mm-es ledekből építene lámpát mindenképpen NYÁKlemezre ültesse a ledeket a szerelhetőség végett.
http://ourworld.compuserve.com/homepages/Bill_Bowden/homepage.htm#menu
http://www.theledlight.com/ledcircuits.html
http://openbookproject.net//electricCircuits/Semi/index.html
LM317+R ÁRAMGENERÁTOR

LM317+R összeszerelve hűtőbordán
- Gondolom a legtöbben a HPL-lámpa méretezésére és üzemeltetésére kíváncsiak, ezért kihagynám ill. másra hagynám az állandóáram-generátor és a feszültségszabályzó IC működési elvének pontos leírását már csak azért is, mert nem szakmám és biztos hogy még a hibáim is rosszul fogalmaznám meg.
Én a legegyszerűbb feszültségstabilizátoros áramgenerátort építettem meg egy LM317T(TO220) szabályozható kimeneti feszültségű stabilizátor IC-vel -továbbiakban IC- és egy ellenállás segítségével a kis ledlámpámban. Léteznek ennél sokkal elegánsabb és jobb megoldások más szabályzó alkatrészekkel de egyszerűbb biztos nincs, ami szerintem egy elektronikában járatlan kertésznek fontos.
- Az igény piros és kék ledekkel való fényelés minimális befektetés mellett, ezért egy átalakított pc tápot használtam egyenáramtápnak. A leadott legnagyobb feszültsége 12V ami kevés, de alacsony igények mellett elégnek is minősülhet ha meg tudunk békélni azzal a ténnyel, hogy minden kör elé építeni kell egy áramgenerátort. Magyarán ha 12V feszültségről dolgozunk 3 piros ledet köthetünk egy körre, 24V esetén pedig 8-at.
- Nos ott tartunk, hogy van egy tápunk és nem tudjuk milyen ledet vásároljunk. Amennyiben még nem volt forrasztópáka a kezedben akkor a legolcsóbb UEC 3W-os csillaghűtőset. Ezt Budapesten a LOMEX-ben tettem, darabonként valahol br.520ft-ért. Méretezésnél a piros ledek nyitófeszültségét 2,5V a kékeket 3,6V-ra vettem, és mindkét ledet 700mA-el kívántam hajtani. A nyitófeszültségek számításhoz szükséges értékét amúgy a gyártó a meghajtó áram függvényében adja meg, fontos azt méretezés és szerelés előtt elolvasni.
- Tehát van 12V-unk, és a piros ledek 2,5V-ot vesznek fel 700mA hajtás mellett.
Gondolhatnánk egy körön elfér 4db, a maradék 2V-ra meg nézünk valami ellenállást. Nem így van, itt lép képbe az IC. Az IC-nek az a szerepe, hogy kisimítsa a hullámzó 12V tápfeszültségünk és egyenletes beállított áramerősséget produkáljon. Ő ezért 3V feszültséget kér üzemi feszültség címén a 12V-ból.
- Máris csak 9V maradt az eredeti 12V-ból, tehát csak 3db piros ledet tudunk egy körre építeni. Ennek az IC-nek az alkalmazásánál érdemes betartani azt a szabályt, hogy minél kevesebb többletfeszültségünk maradjon mert ezt az IC fogja elfűteni, magyarán ne próbáljunk tartós üzemnél 12V-ról egyetlen piros ledet hajtani.
Továbbá érdemes vásárolni egy multimétert kimérni a 12V pontos értékét. Az én ATX-em 12,38V-ot adott le. Esetünkben a többletfeszültség számítása így néz ki:
-
- 12,38V-(3×2,5V+3V)=1,88V
Ezt a feszültséget fűti el az IC.
Most már tudjuk a körünk feszültségesésének adatait, de még nincs egyenletes meghajtó áramunk. Annyit tudunk, hogy maximum 700mA-el hajthatóak ledjeink, ezért erre is méretezünk a körön belül. Az ugye mindenkinek megvan, hogy soros kapcsolásnál a körben futó áram erőssége állandó.
- Mivel az IC a bal és a középső láb között mindig egyenletes 1,25V feszkülönbséget tart fent egy ellenállás közbeiktatásával és az Ohm törvény segítségével egyenletes áramerősséget nyerünk belőle. Ez a lényeg! Mivel a feszültségünk ismert és 700mA az álmunk már csak egy egyszerű R=U/I képlettel megkapjuk a közbeiktatandó ellenállás értékét 1,78Ohm-ra. A következő járatos ellenállásméret az 1,8ohm. Tisztelet MedChem-nek…
A valós áramunk ennek megfelelően 1,25V/1,8ohm=0,695mA
Az ellenállás teljesítménye 1,25V x 0,695mA=0,87W
Így a közbeiktatandó ellenállás 1,8ohm/1W Általánosan elfogadott az a nézet, hogy a számított ellenállásteljesítménytől egyel nagyobb ellenállást alkalmazunk.
- Forrasszuk ezeket egymáshoz a képen látható módon.
Ezzel elkészült a lámpánk első köre. Ugyan ezt a számítási módot alkalmazzuk a kék ledeknél is, csak a ledek nyitófeszültségét 3,6V-ban, vagy a gyári adatoknak megfelelően módosítjuk.
Fontos tudni, hogy a ledek nyitófeszültségei a meghajtó áram függvényében változnak. Minden High power Led vásárlás és alkalmazás előtt át kell nézni a gyári datasheet-et.
Mivel az IC a kör többletfeszültségét elfűti nagyon forró lehet(a sajátom81C), fontos ezt is hűtőbordára szerelni. Ahol vásárolod érdemes kérni hozzá saját hűtőbordát, csavart, szigetelőhüvelyt és szigetelő ásványlapot, mivel az IC saját-hűtőzászlója a középső láb potenciáljával megegyező. Ha a ledek hűtőbordájára szerelnéd megfolyt forrasztóón vagy hibás szigetelésű csillag led esetén nem fog működni a fény. Ha hűtőcsillag nélküli SMD ledet használsz az aljukon található lemez valószínűleg az anóddal azonos potenciálú, így a többi ledet testeli. Szigetelendő.
http://users.telenet.be/davshomepage/current-source.htm
NFET+NPN+2R
Mivel az előző áramgenerátor 3V feszültséget levesz a tápfeszültségünkből alacsony tápfesz esetén ez nagyon hiányozhat. Erre orvosság ez az áramgenerátor ami csak 0,5V-ot igényel. Az elv hasonló csak a különbség annyi, hogy itt a lábak közti feszültségkülönbség csak 0,5V. Hasonlóan az előzőekhez itt is igyekeznünk kell a tápfeszültséget maximálisan lehasználni, ugyanis a maradék feszültséget a FET fogja elfűteni. Érdemes az IC-t hűtőbordára szerelni.
Az NFET működési elvét itt nem boncolgatnám, legyen elég bizonyításnak az, hogy miért is hazudnék pár évvel a nyugdíj előtt. A kör egyszerűbb 2 ellenállásos része csak 20V feszültségig üzemel mivel az NFET kapuja csak ennyit bír el. Még egy ellenállás vagy zéner dióda beiktatásával a kör feszültségviselése 60V-ra nől. Az egyszerűbb 2 ellenállásost 18V feszültségű laptop tápokhoz, a zénerest 24V-os tápra vagy trafókra javaslom 20 V fesz felett.

2 ellenállással 20V feszültségig

3 ellenállással 60V feszültségig
Szükséges alkatrészek a két ellenállásos verzióhoz:
-
- egy NFET (Fairchild FQP50N06L) jele Q2
- egy NPN tranzisztor (Fairchild 2N5088BU)jele Q1
- egy 100kOhm/ 1/4W ellenállás jele R1
- egy méretezett ellenállás amivel a körben folyó áramerősséget határozzuk meg
Ellenállás értéke 1000mA-re:
- R3= 0,5V / 1A = 0,5 Ohm Járatos méret: 0,51 Ohm
R3 teljesítménye: (0,5V x 0,5V) / 0,5 Ohm = 0,5W A hőtűrés miatt 1W-os ellenállást használjunk

Összeszerelt állapotban. A közelebbi ellenállás az R1, a távolabbi az R3. Felül a tranzi, alul az NFET. Huzalok alulról: táp+, LED+, LED-, táp-
- Amennyiben tápunk meghaladja a 20V feszültséget a FET érdekében rövidre kell zárni egy zéner diódával a tranzisztor szélső lábait. A zener érték bármi lehet 20V alatt, válasszunk 5V körülit.
Pl: 1N4732A vagy 1N4733A
Forrás:
http://www.instructables.com
Link:
http://www.instructables.com/id/Circuits-for-using-High-Power-LED_s/?ALLSTEPS
http://www.instructables.com/id/Power-LED_s—simplest-light-with-constant-current/?ALLSTEPS
Gyakorlat
- Fontos tudni, hogy a led hűtőfelületének teljes felületen kell felfeküdnie jó hővezető anyagra, történetesen alumíniumra vagy rézre. Ebből adódóan ez a hordfelület semmi esetre sem lehet ívelt, a ledek a legkissebb felületeltérésre is hőgutával reagálnak még nagy mennyiségű hővezető paszta használata esetén is.
A ledeket én egy processzor hűtőbordájára szereltem ragasztással. Procihűtőt pc-bontóban nézz és 200ft-nál ne adj érte többet. Azért is jó a procihűtő mert nagy felületű hűtést biztosít és nincs olyan amorf alakja mint a legtöbb elektronikai hűtőknek, egy 46mm szélességű nyílásba a growboxon remekül fel tudnak feküdni.
Egy procihűtőn 3 led csillagostól kényelmesen elfért és nem melegedett 27c fölé, ez nyilván a sűrű bordázatnak köszönhető. Mivel a hővezető ragasztó többe kerül mint az egész cucc együtt ezért hővezető pasztát(150-500ft) kentem a csillag aljára, pillanatragasztót meg a sarkaira. Ezt a rögzítés kedvéért írom azoknak akik nem tárolnak állványfúrót a lakásban. Ha épp van kéznél ajánlatos csavarral rögzíteni, de a hővezető paszta itt sem maradhat el. Meg lehet oldani más ragasztóval is de arra figyelj, hogy 120C-t térfogatváltás nélkül elviseljen. Ilyen a legtöbb epoxi műgyanta is, de azoknak nem tudom milyen a hővezetésük, vékonyan kenve biztos jók.
A legjobb megoldás azonban az Artic Silver/Alumina Adhesive hővezető műgyanta alkalmazása. Mindamellett hogy rögzíteni lehet vele kiválló hővezető és szigetelő is egyben. Ez elsősorban az anóddal közös hűtőfelületű ledeknél fontos. A szigetelést meg lehet oldani csillámpapírral vagy szilikonlappal, azonban a rögzítés ebben az esetben bonyolultabbá válhat.
Forrás: http://chfilpo.villamvadasz.hu/drupal/
Hűtőcsillag nélküli ledet az vegyen akinek van már pici gyakorlata forrasztásban, nem egyszerű egy 5mmx4mm-es led 0,5mm-es lábaira bármit ráforrasztani. Nem beszélve arról, hogy az ónmegfolyásból származó hibák nagyobb eséllyel fordulhatnak elő potenciálva a hűtőt. Persze ha saját nyákra szereled ez a hiba nem fordul elő… A hűtőcsillagos ledek mind műanyag lencsések, ezek viszonylag jól bírják a kézi forrasztást, azonban léteznek olyan ledek ahol a diódalap szabadon van, egy rossz mozdulat a pákával és vége.
Kellemes bütykölést mindenkinek.
Végezetül annyit hozzáfűznék, hogy elektronikában teljesen járatlan vagyok ezért minden a cikkben előforduló fogalmazási vagy szakmai hibáért elnézést kérek és kérném, hogy szerkesszétek át.
LEDek használata a növénytermesztésben
A ledek használata a beltéri növénytermesztésben intenzíven bővül, ennek az oka elsősorban a rendkívül hatékony de magas üzemeltetési költségű fémhalogén és nátriumlámpák kiváltásának szándéka. A HID-ek elsősorban direkt megvilágításra alkalmazhatók rövid távolságon hasznosítható fénymennyiségük miatt, a ledeket kiegészítésül is használják a természetes megvilágítás mellett.
A HID-ek kiváltása ledekre legtöbb esetben kudarccal végződik, ennek az oka legtöbbször félreértésekből adódik. Talán külföldi oldalakat bújva találkoztunk már csodát ígérő 100W-os ledlámpát melynek célja a 600W-os HPS kiváltása lenne, azonban a naplókban rendre lehúzzák hozamukat, komoly termesztők hallani sem akarnak ledes fényről. A led feladata nem az, hogy a nátriumlámpa fényáramát produkálja hanem a fotoszintézishez és más növényi életfolyamatokhoz a megfelelő és szükséges hullámhosszú fényt adja le, a kibocsátott fény minden lumene hasznosítható legyen a növény számára.

A “Super” HPS által leadott fény színe
A HID fény színe meg sem közelíti a növény által nagyrészt hasznosítható látható tartományú kék és piros színeket, azonban hatalmas fényintenzitása bőven lefedi a növény igényeit és a hasznosítatlan fénymennyiség sincs káros hatással.
PAR a Wiki szerint
A lényeg itt van, mivel a gyártók csak erősen korlátozott hullámhosszú ledeket adnak ki egyenlőre nincs lehetőség pontosan tervezett kísérletekre melyekkel egy amatőr botanikus megállapíthatná növénye számára ideális színeket, nem tudja senki a pontos színösszetételt a növények palánta, vegetatív és virágzási korában. Persze vannak egészen komolyan vehető próbálkozások, de mindaddig amíg a vásárló nem válogathat a 610-től 760-ig terjedő skálán a piros színű ledekből addig ez csak erősen úttörő vállalkozás lehet. Nyilvánvalóan ugyan ez érvényes a kék skálára is, az infravörös és az UV hasznának megállapítására pedig esély sincs belátható időn belül. A szerencsés amerikai led használók akik viszonylag könnyen hozzájutnak a mélyvörös ledekhez szinte egybehangzóan állítják csodás hatását, legtöbben 1:1 arányú piros:mélyvörös kombinációt ajánl a barkácsolni kedvelő lámpaépítőknek.

A fotoszintézis során a növény által használt fény
A piros színű ledeket a gyártók a 625-630nm hullámhosszon adják ki amelytől való eltérés minimális(10nm körüli), a környezeti hőmérséklet függvénye mely egy konstans hőmérsékletű boxban nem számottevő. Jelenleg csak az Edison és a Ledengin[1] ad ki nagyteljesítményű 660nm hullámhosszú ledet ami a fotoszintézisben dominánsan hasznosul. A kék szín kicsit talán nagyobb szórással jelentkezik a piacon, szinte minden gyártó ad ki 465nm körüli hullámhosszú ledeket, néhány gyártó(pl:Luxeon az új K2-vel) a 440nm királykékkel és a 480nm körüli ciánkékkel kedveskedik a rövid szárcsomók szerelmeseinek.
Azok a termesztők, akik nem sajnálják az időt, pénzt és növényeiket ledekkel megvilágítani a rövid hullámhossz tartományú piros és kék fényüket igyekeznek szélesíteni meleg és hideg fehér fénnyel, jelenleg ez egy jónak tűnő megoldás a piac hiányosságaira. Fontos azonban kihangsúlyozni, hogy a fehér fény csak kiegészítés, tisztán fehér fényű ledekből álló lámpának nagyobb teljesítményűnek kellene lennie mint bármelyik HID. Ha meg azt is észrevesszük, hogy a fehér ledek kibocsátott hullámhossza köszönőviszonyban sincs a PAR görbével hamar lemondunk fehér ledek iránti igényünkről

Szárazföldi növények és zöldalgák pigment spektruma
A fényárammal kapcsolatos félreértésekre is ez adhat magyarázatot, komoly ledelő mély levegőt vesz és elszámol tízig ha csekély hozamát a minimális lumenre fogják társai. Ledes világításnál a fénymennyiség másodlagos, elsődleges a megfelelően kevert színek beállítása és az életfolyamatokhoz való igazítása. Ami idáig kiderült az az, hogy a kék fény a vegetációhoz szükséges és a szárcsomók hosszát befolyásolja, míg a piros fény a virágzásban jut főszerephez. Mivel mindkettőt hasznosítja a növény a fotoszintézisben mint a legfontosabb életfolyamatban érdemes mindkét színt az aratásig alkalmazni csak teljesen eltérő arányokban, míg egy vegetációs korát élő növénynek sok kékre van szüksége a virágzás idején ez háttérbe szorul a pirossal szemben.
Ezen túlmenően még nagyon sok furcsának tűnő próbálkozás is létezik a ledek előnyeinek vizsgálatára, ilyen a teljes nappali szakasz lekövetése a hajnali égbolt és az alkonyat színeinek modellezésétől a sötét éjszakai periódusban tovább világító piros fényekig, mely elmélet szerint a kék fény hiánya indítja be a virágképződést. Véleményem és sokunk véleménye szerint a ledeké a jövő, azonban amíg a piaci felhozatal a különböző hullámhosszok terén hiányosnak mondható addig azt kell mondanunk, hogy a jelen nem a ledeké.

A Procyon100 56db ledet használ 350mA-en hajtva. Az informatikai harvereknél gyorsabban avulnak el a régi technológiájú ledek.
Hasonló félreértésre ad okot a ledek névleges teljesítménye. Sok boldog tengerentúli sporttársunk előszeretettel vásárol névleges teljesítménnyel megadott kész lámpákat miközben nem veszik figyelembe, hogy mind a kék, mind a piros led valódi teljesítménye messze elmarad a névleges teljesítmény mögött, ráadásul azonos típusúak ergo szűk hullámhosszokon fényelnek. A Procyon100 egy 100W-os ledlámpának hazudott sikerdarab odaát mely valódi teljesítménye 50W körül mozoghat. Azért mert a Luxeon azt mondja az első szériás K2-re hogy 3W-os attól még 3,5V a nyitfesz és 350mA-el hajthatod tehát valójában 1W-os ledet használsz. Ha ledet veszel hajtsd ki! A gyártók által megadott áram legtöbbször 350, 700 és 1000mA, azonban a datasheet ismerete nélkül ne állj bele áramgenerátort építeni, a biztonságos maximumot hozd ki a ledekből ha már pénzt adtál értük, csúcsra járatva 3 év alatt kopnak 10%-ot.
Lámpaépítésnél törekedni kell a minél szélesebb kék és piros lefedésre. Kékeknél ne csak az általános kéket(470nm) hanem a királykéket(~450nm) is használjuk, pirosaknál a 625nm, 660nm, 700nm hullámhosszokat is fedjük. Ezen két alapszínt szélesíthetjük további fehér, sárga és narancssárga ledek hozzáadásával. Csak egy vélemény, de indulópontnak jó: a piros:kék:fehér:narancs/sárga 7:2:1:1 felé kellene tartania virágzás esetén. Vegetációnál a piros csökkenthető, a narancs és sárga ledek elhagyhatók.
 Follow
Follow
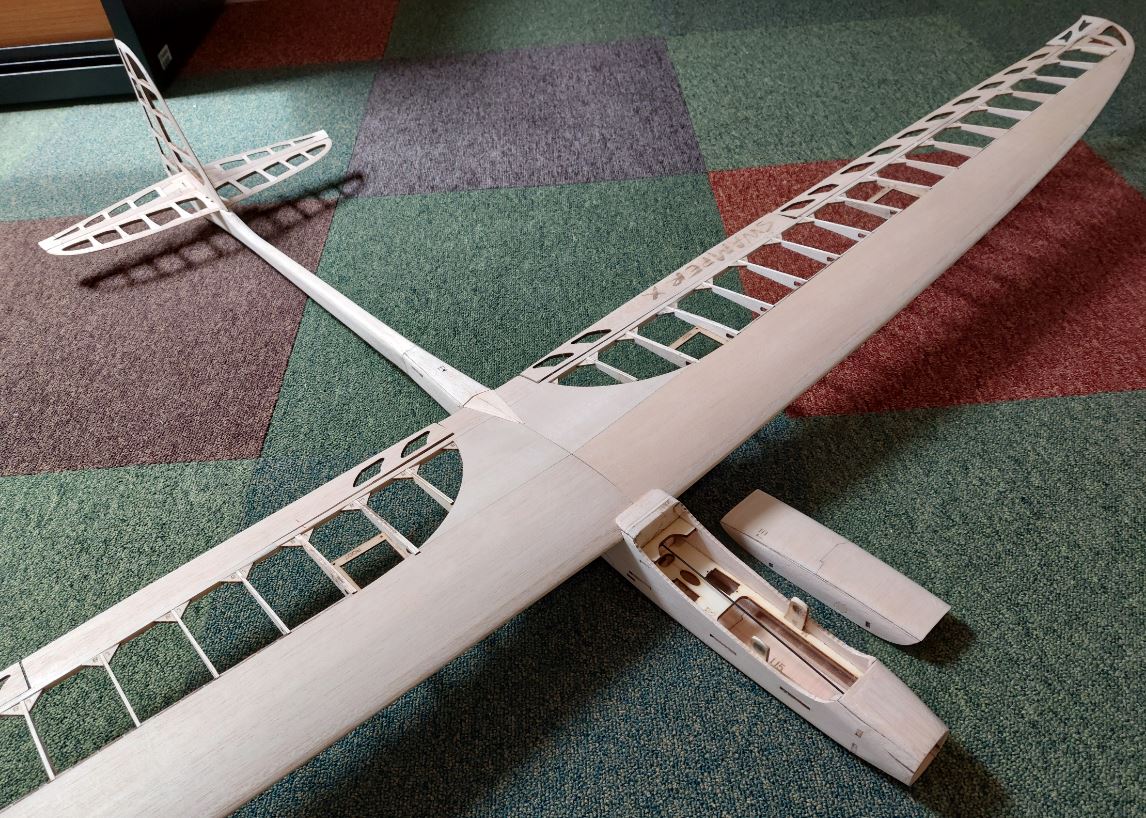
 Az alkatrészeket egy fadobozban kapjuk, ami nemcsak védi, de az előre perforált lemezekből később egy tartót tudunk összerakni, hogy a gépet esetleg ne a párás fűbe kelljen lerakni és a súlypont beállítását is segíti. A teljes kit tartalmazza fa alkatrészeket, szén farokcsövet, tolórudakat és szerelékeket, valamint egy szilikonos felületű nyomtatott építősablont. Ezen nagyon egyszerű a ragasztás.
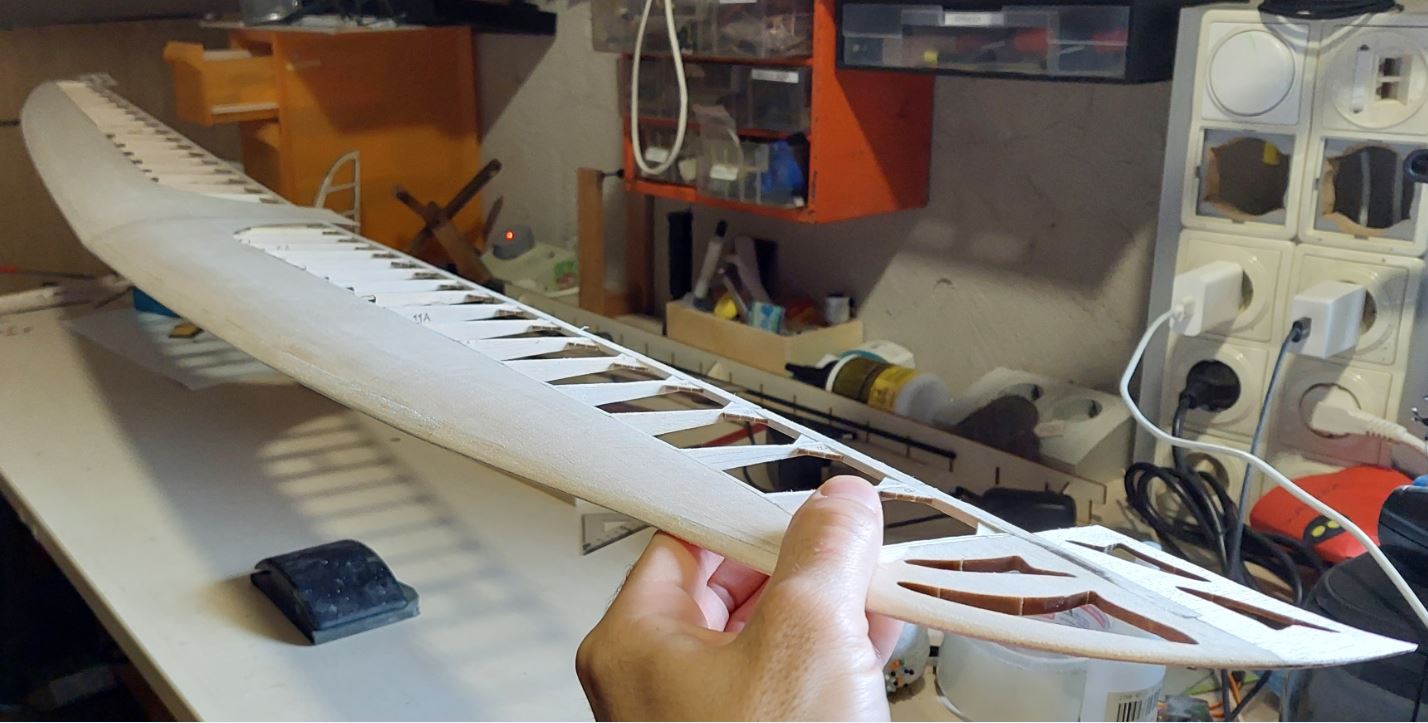
Az alkatrészeket egy fadobozban kapjuk, ami nemcsak védi, de az előre perforált lemezekből később egy tartót tudunk összerakni, hogy a gépet esetleg ne a párás fűbe kelljen lerakni és a súlypont beállítását is segíti. A teljes kit tartalmazza fa alkatrészeket, szén farokcsövet, tolórudakat és szerelékeket, valamint egy szilikonos felületű nyomtatott építősablont. Ezen nagyon egyszerű a ragasztás. Az építést a szárnyal kezdtem. Az S4083 egy enyhén ívelt profil, ezért a pontosabb építés miatt a bordákat fejjel lefelé rögzítjük az építősablonra. A kis lábaikat egyszerűen a megfelelő téglalapokra ragasztjuk egy csepp pillanatragasztóval és egészen addig így dolgozunk, amíg a szárny alsó torziója felkerül. A bordák függőleges rögzítését egy rétegelt sablon is segíti.
Az építést a szárnyal kezdtem. Az S4083 egy enyhén ívelt profil, ezért a pontosabb építés miatt a bordákat fejjel lefelé rögzítjük az építősablonra. A kis lábaikat egyszerűen a megfelelő téglalapokra ragasztjuk egy csepp pillanatragasztóval és egészen addig így dolgozunk, amíg a szárny alsó torziója felkerül. A bordák függőleges rögzítését egy rétegelt sablon is segíti.
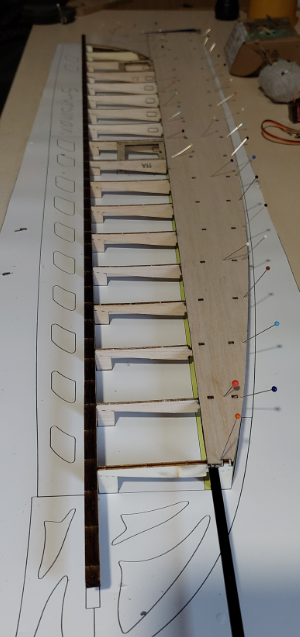






 Sok víz folyt le a Dunána, azóta, hogy szünetelem a modellezést, de a világ nem áll meg. Rengeted fejlődött a modellezők világa, különös tekintettel az elektronikára. Új protokollok, szabványok, és Open Source projektek. Az utóbbiak közül, talán a legnépszerűbb (természetesen OpenTX után :)) a BetaFlight. Mivel hatalmas tudást és ezáltal bonyolultságot a beállításokban hoz magával, készítek egy kis jegyzetet. Lehet másnak is hasznára lesz.
Sok víz folyt le a Dunána, azóta, hogy szünetelem a modellezést, de a világ nem áll meg. Rengeted fejlődött a modellezők világa, különös tekintettel az elektronikára. Új protokollok, szabványok, és Open Source projektek. Az utóbbiak közül, talán a legnépszerűbb (természetesen OpenTX után :)) a BetaFlight. Mivel hatalmas tudást és ezáltal bonyolultságot a beállításokban hoz magával, készítek egy kis jegyzetet. Lehet másnak is hasznára lesz.