Madarak repuleserol
| BIO 554/754 Ornithology Lecture Notes 1 Introduction to Birds |
 |
 |
Birds:
- Kingdom: Animalia, Phylum: Chordata, and Class: Aves
- Similarities between birds & reptiles clearly reveal their evolutionary relationship. However, which Mesozoic reptiles gave rise to birds remains a matter of debate. One hypothesis is that birds evolved from thecodonts (or basal archosaurs). Another, more widely accepted hypothesis, is that birds evolved from small theropod dinosaurs.

A 75-million-year-old meat-eating dinosaur (Bambiraptor feinbergi) has a number of features that look more bird-like
than dinosaur-like, providing evidence that birds may have evolved from dinosaurs.Source: http://exn.ca/dinosaurs/home.cfm?id=20000321-56&SubType=BirdDino
In support of the ‘theropod hypothesis‘:
Evidence against the ‘theropod hypothesis’:
|
In support of the ‘thecodont hypothesis’:
Evidence against the ‘thecodont hypothesis’:
 Source: users.unimi.it/vertpal/galleriafossili/best-rettili /MEGALANCOSAURUS.htm |
Some theropods and other dinosaurs in action
Velociraptors and birds
 |
 The protein was discovered in a femur unearthed in 2003 in the Hell Creek Formation that spans the Wyoming, Montana and the Dakotas in the northwestern U.S. |
— Scientists have confirmed the existence of protein in soft tissue recovered from the fossil bones of a 68 million-year-old Tyrannosaurus rex (T. rex) and a half-million-year-old mastodon. “Not only was protein detectably present in these fossils, the preserved material was in good enough condition that it could be identified,” said Paul Filmer, program director in the NSF Division of Earth Sciences. Schweitzer et al. (2007) discovered soft tissue in the leg bone of a T. rex and other fossils recovered from the Hell Creek sediment formation in Montana. After her chemical and molecular analyses of the tissue indicated that original protein fragments might be preserved, she turned to colleagues John Asara and Lewis Cantley of Harvard Medical School, to see if they could confirm her suspicions by finding the amino acid used to make collagen, a fibrous protein found in bone. Bone is a composite material, consisting of both protein and mineral. In modern bones, when minerals are removed, a collagen matrix–fibrous, resilient material that gives the bones structure and flexibility–is left behind. When Schweitzer demineralized the T. rex bone, she was surprised to find such a matrix because current theories of fossilization held that no original organic material could survive that long. To see if the material had characteristics indicating the presence of collagen, which is plentiful, durable and has been recovered from other fossil materials, the scientists examined the resulting soft tissue with electron microscopy and atomic force microscopy. They then tested it against various antibodies that are known to react with collagen. Identifying collagen would indicate that it is original to T. rex — that the tissue contains remnants of the molecules produced by the dinosaur. “This is the breakthrough that says it’s possible to get sequences beyond 1 million years,” said Cantley. “At 68 million years, it’s still possible.” Asara et al. (2007) successfully sequenced portions of the dinosaur and mastodon proteins, identifying the amino acids and confirming that the material was collagen. When they compared the collagen sequences to a database that contains existing sequences from modern species, they found that the T. rex sequence had similarities to those of chickens, and that the mastodon was more closely related to mammals, including the African elephant. The protein fragments in the T. rex fossil appear to most closely match amino acid sequences found in collagen of present-day chickens, lending support to the idea that birds and dinosaurs are evolutionarily related. “Most people believe that birds evolved from dinosaurs, but that’s based on the ‘architecture’ of the bones,” Asara said. “This finding allows us the ability to say that they really are related because their sequences are related.”

Cross-section through dinosaur bone.
Origin of avian genome size and structure in non-avian dinosaurs — Avian genomes are small and streamlined compared with those of other amniotes, with fewer repetitive elements and less non-coding DNA (a typical bird genome consists of about 1.45 billion base pairs; human genomes are another billion base pairs longer). This condition has been suggested to represent a key adaptation for flight in birds, by reducing the metabolic costs associated with having large genome and cell sizes. However, the evolution of genome architecture in birds, or any other lineage, is difficult to study because genomic information is often absent for long-extinct relatives. Organ et al. (2007) found that bone-cell size correlates well with genome size in extant vertebrates, and used that relationship to estimate the genome sizes of 31 species of extinct dinosaur, including several species of extinct birds. Their results indicate that the small genomes typically associated with avian flight evolved in the saurischian dinosaur lineage between 230 and 250 million years ago, long before this lineage gave rise to the first birds. By comparison, ornithischian dinosaurs were inferred to have had much larger genomes, probably typical of ancestral Dinosauria. Using comparative genomic data, Organ et al. (2007) estimated that genome-wide interspersed mobile elements, a class of repetitive DNA, comprised 5–12% of the total genome size in the saurischian dinosaur lineage, but was 7–19% of total genome size in ornithischian dinosaurs, suggesting that repetitive elements became less active in the saurischian lineage. These genomic characteristics should be added to the list of attributes previously considered avian, but now thought to have arisen in non-avian dinosaurs, such as feathers, pulmonary innovations, and parental care and nesting.
 |
 Haplocheirus sollers |
Bird-dinosaur link strengthened — The fossil record of Jurassic theropod dinosaurs closely related to birds remains poor. Choiniere et al. (2010) reported a new theropod, Haplocheirus sollers (meaning simple, skillful hand), from the earliest LateJurassic of western China represents the earliest diverging member of the enigmatic theropod group Alvarezsauroidea and confirms that this group is a basal member of Maniraptora, theclade containing birds and their closest theropod relatives.It extends the fossil record of Alvarezsauroidea by 63 millionyears and provides evidence for maniraptorans earlier in thefossil record than Archaeopteryx. The new taxon confirms extrememorphological convergence between birds and derived alvarezsauroidsand illuminates incipient stages of the highly modified alvarezsauridforelimb.
Haplocheirus and bird evolution
Related links:
Dinosaur discovery helps solve piece of evolutionary puzzle
Bird-dinosaur link firmed up, and in brilliant technicolor
New dinosaur discovery solves evolutionary bird puzzle
China finds bird-linked dinosaur (with video)
New large-clawed Jurassic dinosaur sheds light on elusive lineage
GWU research team’s dinosaur discovery helps solve piece of evolutionary puzzle

Sinosauropteryx
Fossilized melanosomes and the color of Cretaceous dinosaurs and birds — Spectacular fossils from the Early Cretaceous Jehol Group of northeastern China have greatly expanded our knowledge of the diversity and palaeobiology of dinosaurs and early birds, and contributed to our understanding of the origin of birds, of flight, and of feathers. Pennaceous (vaned) feathers and integumentary filaments are preserved in birds and non-avian theropod dinosaurs, but little is known of their microstructure. Zhang et al. (2010) report that melanosomes (color-bearing organelles) are not only preserved in the pennaceous feathers of early birds, but also in an identical manner in integumentary filaments of non-avian dinosaurs, thus refuting recent claims that the filaments are partially decayed dermal collagen fibers. Examples of both eumelanosomes and phaeomelanosomes have been identified, and they are often preserved in life position within the structure of partially degraded feathers and filaments. Furthermore, these data provide empirical evidence for reconstructing the colors and color patterning of these extinct birds and theropod dinosaurs. For example, the dark-colored stripes on the tail of the theropod dinosaur Sinosauropteryx can reasonably be inferred to have exhibited chestnut to reddish-brown tones.
Related links:
Study offers an insight into dinosaur colors
Feather study highlights dinosaurs’ true colors
In pictures: feathered dinosaur colours identified

Possible scenario for the origin of birds from dinosaurs and the consequent evolution of flight (After Padian 1996.)
Source: http://www.devbio.com/chap16/link1604.shtm

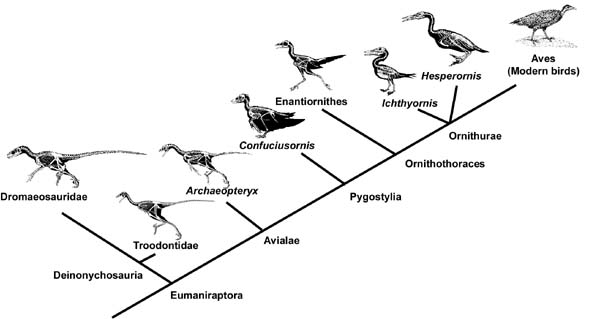
Early birds and dinosaurs. (A) In a simplified cladogram of Theropoda, extant birds (Aves) are nested within Avialae, which currently includes the most recent common flighted ancestor of Archaeopteryx and all of its descendants. The relationships among representative theropod genera Sinosauropteryx, Caudipteryx, Sinornithosaurus, Archaeopteryx, Confuciusornis, Neuquenornis, Yixianornis, Anas, and Gallus are shown. Two major events during the evolution of feathers are noted: the origination of filamentous integumentary structures optimized as homologous with the avian feather and the first appearance of elongate pennaceous feathers. The optimized minimum first appearance of active flight homologous with Aves is also shown. (B) Theropod dinosaurs are one of two clades (with sauropodomorphs) that comprise Saurischia, itself one of the two clades of dinosaurs. (From: Clark and Middleton 2006).
“Archosauria is the clade composed of the most recent common ancestor of birds and their closest living relatives, crocodilians, as well as all of its descendants (see Figure above). On the basis of shared derived morphological characters of the ankle, birds are placed in one of two major lineages of archosaurs, the one that includes both pterosaurs and dinosaurs. Within Dinosauria, birds as a clade are strongly supported by skeletal characters as one lineage of a clade that includes a variety of small raptor dinosaurs. Birds are placed as part of Avialae in the clade Maniraptora, which is part of the progressively more inclusive dinosaurian clades Theropoda and Saurischia. The evolution of both terrestrial and aerial locomotion in the Dinosauria as well as temporal patterns of dinosaur diversification and extinction are the subjects of active research.” – Clark and Middleton (2006).
 With jagged teeth and raptor-like features, the feathered Archaeopteryx is unlike any modern species of bird (Image: G. Mayr/Senckenberg) |
|
A well-preserved Archaeopteryx specimen with theropod features — A nearly complete skeleton of Archaeopteryx with excellent bone preservation shows that the osteology is similar to that of non-avian theropod dinosaurs. This new specimen confirms the presence of a hyperextendible second toe as in dromaeosaurs and troodontids. Archaeopteryx had a plesiomorphic tetraradiate palatine bone (shaped in the same way as in many two-legged dinosaurs) and no fully reversed first toe (or hallux). These observations provide further evidence for the theropod ancestry of birds. In addition, the presence of a hyperextendible second toe blurs the distinction of archaeopterygids from basal deinonychosaurs (troodontids and dromaeosaurs) and challenges the monophyly of Aves (From Mayr et al. 2005). Deinonychosaurs included the famous Velociraptor. Generally, deinonychosaurs were small and lightly built, with deadly teeth and a distinctive sickle-shaped claw on their second toe, which was perfect for disembowelling prey.
| A dromaeosaurid dinosaur may give insights into the evolution of flight (Xu et al. 2000) — Microraptor zhaoianus, the smallest non-avian dinosaur yet discovered, was a bipedal dinosaur that may have been adapted to live in trees. A small section of fuzz, possibly a precursor to feathers, was also found on the specimen. Although it lived some 20 million years after Archaeopteryx, Microraptor is one of the most-bird like dinosaurs known. “(Microraptor) shows a number of modifications to the hips, tail and teeth which are in some ways intermediate between those of advanced meat-eating dinosaurs and birds. “It might represent the most bird-like dinosaur,” Xu said. In addition to being birdlike, Microraptor possessed foot adaptations, including an extended toe, that may have allowed it to grasp the branches of trees. If correct, that would make Microraptor the first known arboreal, or tree dwelling, dinosaur. This tree-dwelling feature may also provide support for the hypothesis that flight evolved from trees, rather from the ground. But, for flight to originate, many scientists say, the animal has to be small, and until now feathered dinosaurs discovered by paleontologists have been too large to fly. Microraptor closes this size gap. The find “further shortens the morphological gaps between dinosaurs and birds,” Xu said. |
 Microraptor zhaoianus Painting by Louis V. Rey |
Source: http://www.geol.umd.edu/~tholtz/G104/10424arch.htm
“Raptors” on the hunt

Model of Microraptor (Dromaeosaurid) – American Museum of Natural History
Source: http://de.wikipedia.org/wiki/Dromaeosauridae

Another possible scenario for the origin of birds.
Source: http://www.geol.umd.edu/~tholtz/G104/10423coel.htm

Evolution of endothermy — Present-day birds are endothermic and, of course, the primitive state among vertebrates is ectothermy. Seebacher (2003) presented a speculated phylogenetic distribution of endothermy among the Dinosauria. Endothermy must have evolved sometime in the lineage leading to modern birds (Ornithurae, very dark shading) and is likely to have occurred in coelurosaurs that exhibited an evolutionary trend toward a decrease in body size, and also lived at mid- to high latitudes (dark shading). It is less likely that endothermy evolved among other theropods that showed an evolutionary trend toward large body size (light gray shading), or among any other group of dinosaurs in which the most recent members attained large body size (very light gray shading). Hypsilophodontids and heterodontosaurids remained small and occurred at mid- to high latitudes, so endothermy may have been of selective advantage in those dinosaurs (gray shading).
 Coelurosauria |
 Dromaeosauridae |
 Rahonavis ostromi (Drawing by T. Michael Keesey) Somewhere between dromaeosaurids & birds |

Archaeopteryx
Bird evolution: a summary — The study of bird origins is over 150 years old. The dinosaurian origin of birds gained broad support after the resemblance between birds and theropods was first recognized by Huxley (1868) and other paleontologists. In Heilmann’s (1926) classic book (“The Origin of Birds”), he suggested that, despite the similarity between birds and theropods, dinosaurs were probably too specialized to be the direct ancestors of birds and proposed that birds and dinosaurs probably evolved from a common ancestor in a group called Thecodontia. Heilmann’s proposal was so authoritative and influential that the thecodont origin of birds became the prevalent hypothesis for nearly half a century. The resurrection of the dinosaurian–bird hypothesis by John Ostrom in the 1970s (Ostrom 1976), with the support of cladistic analysis since the 1980s, has resulted in a general consensus among many paleontologists about the validity of the dinosaurian–bird hypothesis. The discovery of many new and better preserved theropods in the past two decades, particularly those with feather impressions from the Lower Cretaceous of Liaoning, have provided some of the most compelling evidence supporting the hypothesis (Zhou 2004). In more recent years, several additional findings provide yet more support for the dinosaur-bird hypothesis, including molecular evidence of the link between birds and dinosaurs (noted above), the small genomes of birds and saurischian dinosaurs (noted above), similarities in the respiratory systems of birds and theropods, the presence of uncinate processes in both birds and theropods (see below for more details), and similarities between birds and certain theropods in aspects of parental care and nesting. As a result, the growing consensus is that birds are dinosaurs.
| The avian nature of the brain and inner ear of Archaeopteryx (Alonso et al. 2004) – Archaeopteryx, the earliest known flying bird from the Late Jurassic period, exhibits many shared primitive characters with more basal coelurosaurian dinosaurs (the clade including all theropods more bird-like than Allosaurus), such as teeth, a long bony tail and pinnate feathers. However, Archaeopteryx possessed asymmetrical flight feathers on its wings and tail, together with a wing feather arrangement shared with modern birds. This suggests some degree of powered flight capability but, until now, little was understood about the extent to which its brain and special senses were adapted for flight. Alonso et al. (2004) investigated this problem by computed tomography scanning and three-dimensional reconstruction of the braincase of the London specimen of Archaeopteryx. A reconstruction of the braincase and endocasts of the brain and inner ear suggest that Archaeopteryx closely resembled modern birds in the dominance of the sense of vision and in the possession of expanded auditory and spatial sensory perception in the ear. Alonso et al. (2004) concluded that Archaeopteryx had acquired the derived neurological and structural adaptations necessary for flight. An enlarged forebrain suggests that it had also developed enhanced somatosensory integration with these special senses demanded by a lifestyle involving flying ability. |
 |
BBC Video: Short interview with one of the authors, Dr. Milner
Color of an Archaeopteryx feather — Archaeopteryx has been regarded as an icon of evolution ever since its discovery from the Late Jurassic limestone deposits of Solnhofen, Germany in 1861. Carney et al. (2012) report the first evidence of color from Archaeopteryx based on fossilized colour-imparting melanosomes discovered in this isolated feather specimen. Using a phylogenetically diverse database of extant bird feathers, statistical analysis of melanosome morphology predicts that the original colour of this Archaeopteryx feather was black, with 95% probability. Furthermore, reexamination of the feather’s morphology indicates it was an upper major primary covert, contrary to previous interpretations. Additional findings reveal that the specimen is preserved as an organosulphur residue, and that barbule microstructure identical to that of modern bird feathers had evolved as early as the Jurassic. As in extant birds, the extensive melanization would have provided structural advantages to the Archaeopteryx wing feather during this early evolutionary stage of flight.
Links:
Feathered dinosaur had black wings?
Archaeopteryx feather colour and structure revealed
Flying dinosaur had black feathers
- birds are distinguished primarily by feathers; feathers are responsible for two very important features of birds: warm-bloodedness (endothermy) and flight. (Check this site: Animations of Feather Morphogenesis)

| Evolution of feathers — The evolutionary transition series of feather morphologies predicted by the developmental theory of feather evolution (Prum 1999 ). The model hypothesizes the origin and diversification of feathers proceeded through a series derived evolutionary novelties in developmental mechanisms within the tubular feather germ and follicle: |
- Stage I—The origin of an undifferentiated tubular collar and feather germ yielded the first feather, a hollow cylinder.
- Stage II—The origin of differentiated barb ridges resulted in a mature feather with a tuft of unbranched barbs and a basal calamus emerging from a superficial sheath.
- Stage IIIa—The origin of helical displacement of barb ridges and the new barb locus resulted in a pinnate feather with an indeterminate number of unbranched barbs fused to a central rachis.
- Stage IIIb—The origin of peripheral barbule plates within barb ridges yielded a feather with numerous branched barbs attached to a basal calamus. There is insufficient information to establish a sequence for Stage IIIa and Stage IIIb, but both those stages are required in the next stage.
- Stages IIIa+IIIb—The origin of a feather with both a rachis and barbs with barbules created a bipinnate, open pennaceous structure.
- Stage IV—The origin of differentiated proximal and distal barbules created the first closed, pennaceous vane. Distal barbules grew terminally hooked pennulae to attach to the simpler, grooved proximal barbules of the adjacent barb.
- Stage Va—Lateral displacement of the new barb locus by differential new barb ridge addition to each side of the follicle led to the growth of a closed pennaceous feather with an asymmetrical vane resembling modern rectrices and remiges.
- Stage Vb—Division and lateral displacement of the new barb loci yielded opposing, anteriorly and posteriorly oriented patterns of helical displacement producing a main feather and an afterfeather with a single calamus. The afterfeather could have evolved at any time following Stage IIIb, but likely occurred after Stage IV based on modern afterfeather morphology. See Prum (1999) for details of additional stages in the evolution of feather diversity (Stages Vc–f). Also see the ‘Feather evolution‘ page.

Feather evolution – Part 1
Feather evolution – Part 2
Feather evolution – Part 3
Feather evolution – Part 4
Feather evolution and the origin of flight – Part 5

Source: http://www.nurseminerva.co.uk/adapt/feathers.htm

Scanning electron photomicrographs of downy (top) and pennaceous (bottom) barbules
of an American Crow (Corvus brachyrhynchos) (From: Dove et al. 2007).
 |
 When contact angle increases, interfacial tension between liquid and solid (feather) increases. |
Why do (most) feathers repel water? — Wettability of solid surfaces with liquids is governed by the chemical properties and the microstructure of the surfaces. As far as the microstructure of a surface is concerned, fine roughness is well-known to enhance the hydrophobic and hydrophilic properties. A hydrophobic surface where the contact angle for water is enhanced by small roughness and is larger than about 150 degrees is called “superhydrophobic.” The complex structure of most feathers creates such contact angles and makes them hydrophobic (Bormashenko et al. 2007).
| Warm and fluffy — A Chinese fossil shows that primitive feathers covered a small predatory dinosaur from head to tail (Ji et al. 2001). Palaeontologists have found feathers and feather-like structures on several other Chinese dinosaurs, but only on parts of their bodies. This fossil is the first to show feathers over the whole animal, showing that dinosaurs may well have evolved feathers for insulation before they were used for flight. “This is the specimen we’ve been waiting for,” said Ji Qiang of the Chinese Academy of Geological Sciences. About a half-meter long, the fossil was a juvenile dromaeosaur, a close relative of Velociraptor and a member of the theropod family. Downy fibres covered its head and tail, and tufts of filaments that resemble primitive feathers sprouted from other parts of the body. Branched structures like modern feathers grew on the backs of the animal’s arms. The long rigid tail and other skeletal features mark the fossil as a dinosaur rather than a bird. The Chinese-American team verified that the top and bottom slabs which sandwiched the bones matched exactly to assure it was not a fake. The 130-million-year old fossil “shows us that advanced theropod dinosaurs may have looked more like weird birds than giant lizards,” says Mark Norell, a palaeontologist at the American Museum of Natural History in New York. — Jeff Hecht, New Scientist |  Photo: Mack Blison, American Museum of Natural History |

Epidexipteryx hui. a, Main slab; b, c, skull in main slab (b) and counterslab (c); d, four elongate ribbon-like tail feathers; b’, c’, line drawings of b and c, respectively. Abbreviations: l1, l2 and l7, 1st, 2nd and 7th left teeth of upper jaw; l1′, r1′ and r5′, 1st left, 1st right and 5th right teeth of lower jaw; l2 and r2, 2nd left and right teeth of upper jaw.
Jurassic maniraptoran with elongate ribbon-like feathers — Recent coelurosaurian discoveries have greatly enriched our knowledge of the transition from dinosaurs to birds, but all reported taxa close to this transition are from relatively well known coelurosaurian groups. Zhang et al. (2008) reported a new basal avialan, Epidexipteryx hui, from the Middle to Late Jurassic of Inner Mongolia, China. This new species is characterized by an unexpected combination of characters seen in several different theropod groups, particularly the Oviraptorosauria. Phylogenetic analysis shows it to be the sister taxon to Epidendrosaurus, forming a new clade at the base of Avialae. Epidexipteryx also possessed two pairs of elongate ribbon-like tail feathers, and its limbs lack contour feathers for flight. Epidexipteryx‘s ribbon-like tail feathers could have served as ornamentation as well as balancing tools for help with moving along tree branches. Shorter feathers also covered the dinosaur’s body and could have served as insulation. This finding shows that a member of the avialan lineage experimented with integumentary ornamentation as early as the Middle to Late Jurassic, and provides further evidence relating to this aspect of the transition from non-avian theropods to birds.

Epidexipteryx hui
Blue Whistling-Thrush
 Blue Whistling-Thrush (Myiophonus caerulea) © Dr. Bakshi Jehangir – www.birdsofkashmir.com  Transmission electron micrographs of the spongy medullary keratin of the UV-colored feather barbs of Myiophonus caeruleus. Left panel: cross-section of a UV-colored feather barb ramus showing the solid keratin of the barb cortex at the periphery, three adjacent medullary cells with spongy keratin matrix and cell walls, and melanosomes around the large vacuole at the center of the barb ramus. Right panel: close-up of the spongy medullary matrix of keratin bars and air vacuoles. Scale bar equals 500 nm. Abbreviations: c = barb cortex; cw = cell wall, k = spongy medullary keratin, m = melanosome, and v = air-filled vacuole. |
Avian Plumage Color (Prum et al. 2003) — The colors of avian plumage are produced by chemical pigments (e.g., melanin or carotenoids) or by nanometer-scale biological structures that differentially scatter, or reflect, wavelengths of light. No exclusively blue or UV-colored pigments are known in vertebrates, but various carotenoid pigments in bird feathers produce UV wavelengths in combination with human-visible yellow, orange, or red colors. Ultraviolet structural colors of feathers can be produced by two types of structures. Primarily iridescent colors are produced by arrays of melanin granules in feather barbules. Those structural colors are created by coherent scattering, or constructive interference, of light waves scattered from the layers of melanin granules in barbules. A few species of hummingbirds and European Starlings are known to produce UV hues with coherently scattering melanin arrays in feather barbules. The most commonly distributed UV hues, however, are structural colors produced by light scattering from the spongy medullary layer of feather barbs. To date, primarily UV hues have been documented in the feather barbs of Chalcopsitta cockatoos (Psittacidae) and Myiophonus thrushes (Turdidae). Extensively UV hues with a peak reflectance in the human-visible blue range have been observed in feather barbs of Blue Tits (Parus caeruleus), Bluethroats (Luscinia svecica), and Blue Grosbeak. In addition, Prum et al. (2003) have found extensive UV reflectance from apparently blue feather barbs in many families and orders of birds including motmots (Momotidae), manakins (Pipridae), cotingas (Cotingidae), fairy wrens (Maluridae), bluebirds (Sialia), buntings and others. The structural UV hues of feather barbs, like other barb structural colors, are produced by the keratin air matrix of the spongy medullary layer of the barb ramus. However, the precise physical mechanism by which the human-visible and UV barb colors are produced remains controversial. Analysis of the spongy medullary keratin of UV-colored feather barbs of Myiophonus caerulea by Prum et al. (2003) demonstrated that, in this species, color-producing tissue is substantially nanostructured at the appropriate spatial scale to produce the observed ultraviolet hues by coherent scattering, or constructive interference. |
The iridescent plumage of hummingbirds

Color patterns of feathers. (A) Representative patterns within feathers. (B) Some other basic patterns such as bars, circles, and spots.
(C) There are also, of course, color patterns at the level of the entire body (From: Yu et al. 2004).
- Other important characteristics of birds include:
- forelimbs modified as wings
- feathered tail
- toothless horny beak


A. The relationship between select theropods and tail reduction in bird evolution.
B. Evolution of short-tailed birds exemplified by Archaeopteryx, Iberomesornis, and Columba (pigeon)
tail vertebrae. Note the reduction in number of vertebrae and centrum (body) length. Both Iberomesornis
and Columba possess a pygostyle (asterisk). Scale bar = 2 cm (Gatesy and Dial 1996).

Golden Eagle skull
Source: http://www.azdrybones.com/birds.htm
The loss of teeth in birds (from Louchart and Viriot 2011) — The Cenozoic bird fossil record (65.5 million years ago to present) contains only toothless Neornithes. By contrast, most Mesozoic birds (146 to 65.5 million years ago) had teeth. Thus, edentulism (complete loss of teeth) in Neornithes occurred between about 125 and 65.5 million years ago. The acquisition of a muscular gizzard and of a rhamphotheca appear to have been crucial in allowing edentulism and making it viable. Food is stored in the crop, and hence continuously available even outside feeding activities. The muscular gizzard efficiently processes this food, allowing the continuous provision of abundant nutrients necessary for the high metabolic demands of flight. Together with many morphological changes, such as lightening of the skeleton, skeletal structure reinforcements and fusions, and displacement of the center of gravity, higher metabolic rates allowed the improvement and diversification of sustained powered flight. Homeothermy and sustained powered flight arose in an indirect link with the whole process of tooth loss in birds, and with other innovations.

Proposed evolutionary interactions related to the loss of teeth in birds. Several major morphological, physiological and behavioral innovations favored or made possible (arrows) the evolution of other innovations in a complex way: some facilitated edentulism in birds, whereas others led to avian evolutionary success following, and despite, tooth loss, as the Aves are the most speciose class of extant tetrapods. Dashed arrows represent less obvious influences. The horizontal distribution of events reflects approximately their relative temporal occurrences, when known, although some cannot be assigned to a well-defined relative placement.
The loss of teeth in birds allowed for unprecedented diversification of rhamphothecae in terms of size and shape. The diversity in beak shapes and functions in extant birds exceeds by far that observed in the jaws or snout of all other tetrapods, and involves slender or light architectures, extremely varied shapes and curvatures, and specialized kineses that would have been impossible with dentition. By contrast, Mesozoic birds that retained teeth show only a limited diversity of shapes of the snout or incipient beak. The evolution of diverse extreme beak shapes was completed during the first half of the Cenozoic, following tooth loss, in pelicans, stork-like birds, duck-like and flamingo-like taxa, birds of prey, wide-gaped and short-beaked aerial insectivores, and even hummingbirds. The rhamphotheca proves at least as efficient as teeth for food acquisition, whether it is smooth or serrated. Beaks also took on additional functions secondarily, such as feeding young, preening, grooming, courtship and display, communication, and even tool manufacture and manipulation. Such functions have probably contributed to the success of the Neornithes.
- birds vary substantially in size, ranging from the Bee Hummingbird (Mellisuga helenae; 5 – 6 cm long & weighing less than 2 grams) to the flightless Ostrich (up to 2.5 m tall & 140 kg in weight). The largest flying birds (teratorns) are, unfortunately, extinct.

Source: sped2work.tripod.com/evidence.html
A South American ‘terror bird’ (a Phorurhacoid).
This extinct group of predatory, flightless birds dominated South America from
65 million to 2.5 million years ago. The largest known terror-bird species grew nearly 10 feet
(3 meters) tall and weighed 1,100 pounds (500 kilograms).
Terror Birds: Reconstructing behavior from CT scanning & modern hawk biology
 Bee Hummingbird Source: http://www.markuskappeler.ch/tex/texs/bienenelfe.html  Nature Video – Hummingbirds: Magic in the Air Nature Video – Hummingbirds: Magic in the Air
|
 Ostrich |
Why so many small birds? — Birds range about 41,000-fold in body size from the tiny 2-g Bee Hummingbird (Calypte helenae) to
Ostriches (Struthio camelus) that can weigh over 100 kg. However, more than half of all bird species weigh less than 38 g. Generally, small-bodied
species are also more abundant (more individuals) than large-bodied species. The same patterns have been documented for several groups of
organisms, e.g., snakes and mammals, which suggests that there is a general reason why there are so many small species. The very unequal distribution of
body sizes in evolutionary lineages could be the outcome of biased evolution, with natural selection favoring small size. This hypothesis has received a lot of
discussion in the recent literature, but has thus far not has been convincingly demonstrated. Another possibility is that small-bodied species speciate faster.
However, statistical analyses accounting for historical relatedness of present-day species indicate no relation between body size and the rate of speciation.
Finally, instead of little by little, the dominance of small species may have arisen suddenly, when approximately 65 million years ago (presumably) a large
meteorite hit the earth, causing mass extinctions. However, analysis of body sizes and genetic differences of extant species reveals that while avian species
numbers were approximately halved, the catastrophe affected small and large species equally. Thus, the reason why most species are small does not seem to
be due to differential rates of speciation or extinction. Rather, the cause appears to be in the tempo and mode of evolution. Analyses of the body sizes of living
birds suggest that most differences in body size between species arise at the moment of speciation. Differences between small-bodied species are smaller
than between large-bodied species and this difference probably also has its origin at the moment of speciation. Consequently, groups of small species stay
small, whereas groups of large species are more variable in body size, so that in the end most species are small (Bokma 2002, Bokma 2004).
Despite variation in size, all living birds exhibit a remarkable similarity because of their (or their ancestor’s) adaptations for flight. The success of birds, as a group, is in large part due to this ability to fly! Flight is, however, demanding and the bird body shows several modifications for this mode of locomotion, including lightness, streamlining (see European Starling below), strength (rigid skeleton and strong, efficient muscles), and efficient energy utilization

A European Starling in a wind tunnel with an airflow of
9 m/s visualized using the smoke-wire technique.
(Source: http://www.biology.leeds.ac.uk/staff/jmvr/Flight/fvbzwjm.htm)\
The Life of Birds by David Attenborough – To Fly or Not to Fly
The skeleton of birds shows numerous modifications for the demands of flight:

- bones are generally light relative to their size &, in many species, are pneumatic (filled with air spaces but reinforced with internal struts or trabeculae)
- large, soaring birds (e.g., albatrosses, vultures, & hawks) have pneumatic bones
- divers (e.g., loons) must be ‘heavier’ to dive & so have bones that are less pneumatic (see example below) or not pneumatic
- small birds (e.g., many passerines) do not have pneumatic bones (their bones are so small that little weight would be ‘saved’ with pneumatic bones)


Schematic cross-section through a bird bone.
A – periosteal surface, B – lamellar cortical layer,
C – endiosteal surface, D – trabecular layer,
E – pores/pneumatic openings/blood vessel openings
(From: Davis 1998).

Vertebrae of a Wood Duck (Aix sponsa) and a Ruddy Duck (Oxyura jamaicensis) showing the outer layers of compact cortical bone surrounding the trabecular bone. The vertebrae of Ruddy Ducks have more compact bone and less trabecular bone. Ruddy Ducks are diving ducks whereas Wood Ducks are dabbling ducks that forage at or near the water’s surface. More compact bone and less trabecular bone makes bones, and diving ducks, heavier and, therefore, makes them more efficient divers (Figure from Fajardo et al. 2007).
- loss of bones, fusion of bones, & tight articulations to reduce weight and increase strength, e.g.:
- loss of teeth
- thoracic region:
- tightly articulated vertebrae
- ribs with upper (vertebral) & lower (sternal) segments that connect the vertebral column with the sternum
- uncinate processes overlap successive ribs to reinforce rib-cage. They also play an important role in respiration.

Representative skeletons showing the morphological differences in the rib cage associated with different forms of locomotion in (A) a walking species, Cassowary (Casuaris casuaris); (B) a non-specialist, Eagle Owl (Bubo bubo); and (C) a diving species, Razorbill (Alca torda). Uncinate processes are short in walking species, of intermediate length in non-specialists and long in diving species. In all photographs cranial is to the left; scale bar, 5 cm.
Functional significance of the uncinate processes in birds — Uncinate processes are bony projections that extend from the vertebral ribs ofmost extant birds. In 1935, Zimmer (1935) postulated thatthe uncinate processes played some role during inspiration. Other hypotheses have linked these processes withstiffening or strengthening the rib cage or providing attachment sites for muscles stabilizingthe shoulder. Recent electromyographic studies of Giant Canada Geese confirmed Zimmer’shypothesis by demonstrating that these processes are integral componentof the ventilatory mechanics of birds being involved in both inspirationand expiration (Codd et al. 2005). The processes are associatedwith fleshy parts of the Mm. intercostales externi, the Mm.appendicocostales that originates from the proximal edge ofthe uncinate and inserts onto the following vertebral rib. The Mm. appendicocostales is active during inspirationin Giant Canada Geese, suggesting the processes facilitatethe craniad movement of the ribs, which would in turn move thesternum ventrally. The base of the uncinateprocesses serves as a brace for the insertions of the `finger-like’projections of the M. externus obliquus abdominus that pullthe sternum dorsally during expiration.Given that the processes provide attachment sites for theseimportant respiratory muscles, any change in uncinate morphologymay have a significant effect on ventilation.
Tickle et al. (2007) derived a model demonstrating thatuncinates act as levers that improve the mechanical advantagefor the forward rotation of the dorsal ribs and therefore loweringof the sternum during respiration. The length of these processesis functionally important; longer uncinate processes increasingthe mechanical advantage of the Mm. appendicocostales muscleduring inspiration. Morphological studies of four bird speciesshowed that the uncinate process increased the mechanical advantageby factors of 2–4. An examination of variation in skeletal parametersin birds with different primary modes of locomotion (non-specialists,walking and diving) revealed that uncinate length is more similar in birds thathave the same functional constraint, i.e. specialization toa locomotor mode. Uncinate processes are short in walking birds,long in diving species and of intermediate length in non-specialist birds.These results demonstrate that differences in the breathingmechanics of birds may be linked to the morphological adaptationsof the ribs and rib cage associated with different modes oflocomotion.
-
- pelvic region:
-
-
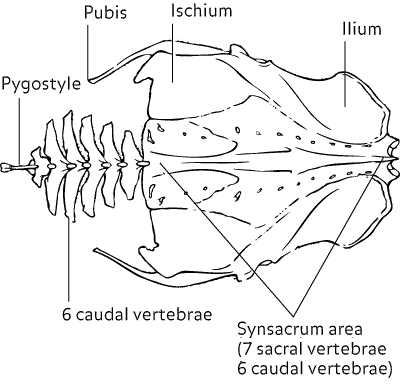
- pelvic (innominate) bones are fused with lumbar & sacral vertebrae. The fused portion of the vertebral column is called the synsacrum & it’s composed of the last thoracic vertebra, the lumbars, sacrals, & anterior caudals.
- last few caudal vertebrae are partially fused to form the pygostyle (that helps support the tail feathers)
-
-
- forelimbs:
- ulna (the bone that supports the secondaries) is enlarged
- carpal bones (wrist bones) are reduced in number (just 2)
- metacarpals (palm bones) – 1st & 5th metacarpals are lost; 2nd, 3rd, & 4th are united (with vestigial carpals) to form the carpometacarpus
- digits – only 3 (rather than the typical 5 found in most vertebrates)
- phalanges (the bones that make up the digits) – few in number; 4 – 7 make up the 3 digits
- forelimbs:

The forelimbs of another flying vertebrate (lesser short-nosed fruit bat, Cynopterus brachyotis)
are very different from those of birds (and now-extinct reptiles, pterosaurs, could also fly).

Aerodynamic efficiency: birds vs. bats — Flight is one of the energetically most costly activities in the animal kingdom, suggesting that natural selection should work to optimize flight performance. The similar size and flight speed of birds and bats may therefore suggest convergent aerodynamic performance; alternatively, flight performance could be restricted by phylogenetic constraints. Muijres et al. (2012) tested which of these scenarios fit to two measures of aerodynamic flight efficiency in two passerine bird species (Pied Flycatcher and Blackcap) and two New World leaf-nosed bat species. Using time-resolved particle image velocimetry measurements of the wake of the animals flying in a wind tunnel, the span efficiency, a metric for the efficiency of generating lift, and the lift-to-drag ratio, a metric for mechanical energetic flight efficiency, were derived. Birds significantly outperformed the bats in both metrics, likely due to variation in aerodynamic function of body and wing upstroke: Bird bodies generated relatively more lift than bat bodies, resulting in a more uniform spanwise lift distribution and higher span efficiency. A likely explanation would be that the bat ears and nose leaf, associated with echolocation, disturb the flow over the body. During the upstroke, the birds retract their wings to make them aerodynamically inactive, whereas the membranous bat wings generate thrust and negative lift. Despite the differences in performance, the wake morphology of both birds and bats resemble the optimal wake for their respective lift-to-drag ratio regimes. This suggests that evolution has optimized performance relative to the respective conditions of birds and bats, but that maximum performance is possibly limited by phylogenetic constraints. Although ecological differences between birds and bats are subjected to many conspiring variables, the different aerodynamic flight efficiency for birds and bats may help explain why birds typically fly faster, migrate more frequently, and migrate longer distances than bats.
| Bird embryos have 5 fingers — The developmental origin of digits in the wings of birds has been hotly debated for more than a century. Larsson and Wagner (2002) have shown unequivocally that five digits are present during the early development of chickens. The earliest stage of digits is a condensation of mesenchymal cells and digit I is, thus, transiently present during development. This establishes that three digits in the wings of birds are digits II–IV. However, theropod dinosaurs are assumed to have had digits I–III. Feduccia & Nowicki (2002) claim that for this reason, a descent of birds from theropods is impossible and that instead, birds are descended from archosaurs other than dinosaurs (e.g., thecodonts). Galis et al. (2002) believe it improbable that the multitude of shared characters between theropods and birds are the result of convergence. That leaves three possible scenarios: (1) birds descending from archosaurs other than dinosaurs, which cannot satisfactorily explain the many similarities between birds and theropods; (2) the ‘frame shift hypothesis’ [theropod ancestors of birds initially had digits I–III and, before the origin of birds, a shift occurred such that digits II–IV developed with identities I–III; Wagner and Gauthier (1999)] for which there is as yet no adaptive significance that would overcome the evolutionary constraint; and (3) birds descending from theropods with digits II–IV, which is the most parsimonious evolutionary transition scenario but for which there is as yet no fossil evidence. |
 Developmental stages of chick wings in dorsal view. (a) Adult wing with three ossified digits. (b) Stage 35 embryo with four chondrified digits. (c) Stage 29 embryo with five mesenchymal digits (From: Galis et al. 2002). |
-
- hindlimbs:
- proximal tarsals are fused with the tibia to form the tibiotarsus & distal tarsals are fused with metatarsals to form the tarsometatarsus
- hindlimbs:

Source: trc.ucdavis.edu/mjguinan/apc100/modules/Musculoskeletal/skeleton/limb3/limb.html

Avian toe claws — Glen and Bennett (2007) placed birds into six categories (GB, Gg, Ga, Ag, Aa and V) based the degree of ground or tree foraging; GB = ‘ground-based’ birds, limited to foraging on the ground; Gg = ‘dedicated ground foragers’; Ga = ‘predominantly ground foragers’; Ag = ‘predominantly arboreal foragers’; Aa = ‘dedicated arboreal foragers’; V = ‘vertical surface foragers’. Analysis of the toe claws of 249 species of birds revealed that claw curvature increases as tree foraging becomes more predominant.
-
- sternum
-
-
- ‘flat’ in flightless birds (like ostriches & rheas) but a large keel (site of attachment of the large flight muscles) is present in most birds
-

Sword-billed Hummingbird (Ensifera ensifera) skeleton
(Used with permission of Dennis Paulson, Director, Slater Museum of Natural History)

Rhea (Rhea americana) skeleton
Source: http://www.uiowa.edu/~fyi/issues2000/03232001/bird.html
-
-
- articulates with the coracoids which, in turn, articulate with the clavicles & scapulae to provide support needed to withstand forces generated by flight muscles during flight
-

Uncinate processes (arrows) of (a) a running bird, the Cassowary (Casuaris casuaris), (b) a flying bird, the Eagle Owl (Bubo bubo), (c) a diving bird, the Razorbill (Alca torda); analysis indicates that uncinate processes are shorter in running, long in diving, and intermediate in all other birds, (d) Oviraptor philoceratops, (e) Velociraptor mongoliensis. Anterior is to the right in all figures. Scale bars, 5 cm.
Dinosaurs and birds share uncinate processes — In 1868, Thomas Huxley first proposed that dinosaurs were the direct ancestors of birds and subsequent analyses have identified a suite of ‘avian’ characteristics in theropod dinosaurs. Ossified uncinate processes are found in most species of extant birds and also occur in extinct non-avian maniraptoran dinosaurs. Their presence in these dinosaurs represents another morphological character linking them to Aves, and further supports the presence of an avian-like air-sac respiratory system in theropod dinosaurs, prior to the evolution of flight. Codd et al. (2007) conducted a phylogenetic analysis of the presence of uncinate processes in Aves and non-avian maniraptoran dinosaurs and found they were homologous structures. Furthermore, recent work on Canada Geese has demonstrated that uncinate processes are integral to the mechanics of avian ventilation, facilitating both inspiration and expiration. In extant birds, uncinate processes function to increase the mechanical advantage for movements of the ribs and sternum during respiration. The study by Codd et al. (2007) presents a mechanism whereby uncinate processes, in conjunction with lateral and ventral movements of the sternum and gastral basket, affected avian-like breathing mechanics in extinct non-avian maniraptoran dinosaurs.
The muscles of birds have also been modified by natural selection to meet the demands of flight:
- reduction in some muscles to minimize weight:
- jaw muscles are reduced in many birds (powerful muscles often unnecessary because food is swallowed whole or in large pieces, e.g., owls)
- hindlimb muscles reduced in many species because:
- the rigid skeleton of birds (hindlimb, pelvic girdle, & synsacrum) provides much support &, as a result, less musculature is needed
- hindlimbs are sometimes used for little else but perching (e.g., hummingbirds & swifts)
- flight muscles (pectoralis & supracoracoideus) are very large & located near the center of gravity:
- pectoralis or downstroke muscle – originates on the keel and inserts on the underside of the humerus
- supracoracoideus or upstroke muscle – originates on the keel and inserts on the upper side of the humerus


Tendon of the supracoracoideus passing through the
foramen triosseum and inserting on the humerus
(From: Degernes and Feduccia 2001).

Right wing of an Atlantic Puffin. c, coracoid; f, furcula, h, humerus, LD, latissimus dorsi muscle;
r, radius; s, scapula; SC, supracoracoideus tendon; SHC, scapulohumeralis caudalis muscle;
st, sternum; TF, triosseal foramen or canal; TS, triceps scapularis muscle; u, ulna
(From: Kovacs and Meyers 2000).
| Migration & muscle damage — Exercise-induced muscle damage is often a consequence of strenuous exercise. In birds, the high intensity and long duration of migratory flights could result in significant muscle damage, possibly due to metabolic factors (e.g., elevated temperature, lowered pH, & ionic shifts). Because exercise-induced muscle damage is characterized by leakage of muscle-specific proteins into the blood plasma (e.g. creatine kinase), Guglielmo et al. (2001) used plasma creatine kinase (CK) activity as an indicator of muscle damage to determine if the high intensity, long-duration flights of two migratory shorebirds cause damage that must be repaired during stopover. They found that plasma CK activity was significantly higher in migrating Western Sandpipers (a non-synchronous, short-hop migrant) than in non-migrants. Similarly, for Bar-tailed Godwits (a synchronous, long-jump migrant), plasma CK activity was highest immediately after arrival from a 4000–5000 km flight from West Africa to The Netherlands, and declined before departure for arctic breeding areas. Juvenile Western Sandpipers making their first southward migration had higher plasma CK activity than adults. These results indicate that muscle damage does occurs during migration, and that it is exacerbated in young, relatively untrained birds. However, increases in plasma CK activity were relatively small, suggesting limited muscle damage. Thus, avian flight muscles appear to be superbly adapted to high intensity exercise, and likely possess morphological, physiological and biochemical mechanisms to prevent damage (e.g. antioxidants). |  |

Changes in pectoral muscle size due to simulated raptor attack compared with control treatment (gull),
shown for each of five trials. The direction of each arrow reflects the treatment order (predator after gull or vice versa).
Ruddy Turnstones build pectoral muscle after raptor scares — To cope with changes in the environment, organisms not only show behavioural but also phenotypic adjustments. This is well established for the digestive tract. Van den Hout et al. (2006) described the first case of birds adjusting their flight machinery in response to predation risk. In an indoor experiment, Ruddy Turnstones (Arenaria interpres) were subjected to an unpredictable daily appearance of either a raptor or a small gull (as a control). Ruddy Turnstones experiencing threat induced by a flying raptor model, longer than after similar passage by the gull model, refrained from feeding after this disturbance. Pectoral muscle mass, but not lean mass, responded in a course of a few days to changes in the perceived threat of predation. Pectoral muscle mass increased after raptor scares. Taking the small increases in body mass into account, pectoral muscle mass was 3.6% higher than aerodynamically predicted for constant flight performance. This demonstrates that perceived risk factors may directly affect organ size.
- hindlimb (leg) muscles – concentrated on the proximal portion of the hindlimbs to keep weight near the center of gravity
 |
 |
 |
Dorsoplantar (center) and lateral (right) views of the intertarsal joint: ct, cranial tibial tendon;
fl, fibularis longus tendon; lde, long digital extensor tendon; g, gastrocnemius tendon; and t, tibial cartilage
(center & right images from: Linn et al. 2003).
Summary – Avian anatomical adaptations for flight:
- streamlined body (reduced resistance when moving through the air)
- feathers (light and help produce the streamlined body)
- bones (pneumatic & reduced in number to reduce weight; fused in some cases for increased strength)
- muscles & viscera (centralized to keep most of a bird’s mass near center of gravity)
- forelimbs modified as wings (airfoil to generate lift)
| Avian personalities — Personalities are general properties of humans and other animals. Different personality traits are phenotypically correlated, and heritabilities of personality traits have been reported in humans and various animals. In Great Tits, consistent heritable differences have been found in relation to exploration, which is correlated with various other personality traits. van Oers et al. (2004) examined whether or not risk-taking behavior is part of these avian personalities. They found that (1) risk-taking behavior is repeatable and correlated with exploratory behavior in wild-caught hand-reared birds, (2) in a bi-directional selection experiment on ‘fast’ and ‘slow’ early exploratory behavior, bird lines tend to differ in risk-taking behavior, and (3) within-nest variation of risk-taking behavior is smaller than between-nest variation. To show that risk-taking behavior has a genetic component in a natural bird population, van Oers et al. (2004) bred Great Tits in the lab and artificially selected ‘high’ and ‘low’ risk-taking behavior for two generations. They found a realized heritability of 19.3% for risk-taking behavior. With these results, the authors show that risk-taking behavior is linked to exploratory behavior, and provide evidence for the existence of avian personalities. | Risk-taking behavior was also found to be correlated with other aspects of avian personality. Novelty, exploration and risk-taking behaviors seem to be traits of the personality concept, which is in line with the results of other studies on personalities. Risk-taking behavior is known to influence life-history decisions, and evidence is also accumulating that other personality traits affect reproduction, survival and dispersal. In sum, birds have genetically determined personalities that can be observed in a variety of ecological circumstances. |
Birds live longer! –Like mammals, birds exhibit a rough positive correlation between body mass and maximum recorded life span. However, at any specific body mass, birds average some  2 to 3 times the longevity of mammals. Specific avian groups are even longer-lived than this overall average. Long-lived birds such as macaws, which weigh 500 to 1000 g, may occasionally live as long as 100 years (and the oldest known living bird, as of 2003, was a 51-year-old Laysan Albatross; photo of a Laysan Albatross to the right was taken by Chandler Robbins). Birds achieve these remarkable life spans despite possessing several traits that modern theories of aging suggest should make them substantially shorter-lived than mammals. First, reactive oxygen species, which are highly damaging molecules produced as a normal by-product of aerobic metabolism, are now thought to contribute substantially to the generalized degenerative changes of aging across a wide spectrum of species. An index of exposure to such molecules is thought to be lifetime oxygen consumption per cell in an organism. Yet oxygen consumption per unit time per cell of birds can range as high as 2 to 2.5 times that of mammals. So when this level of oxygen consumption is combined with the long lives of birds, one may postulate that lifetime exposure to reactive oxygen species in long-lived avian cells may be 10 to 20 times that of short-lived mammals such as mice and 2 to 5 times that of even long-lived mammals such as humans. Clearly birds have evolved some type of especially effective mechanisms for protecting against the accumulation of oxidative damage. The details of these protective measures remain elusive. It may be that because of more effective electron scavenging in avian mitochondria, birds produce fewer reactive oxygen species per unit of oxygen consumption or that birds have more active enzymes for detoxifying these molecules. However, research into these areas has so far been limited (Austad 1997).
2 to 3 times the longevity of mammals. Specific avian groups are even longer-lived than this overall average. Long-lived birds such as macaws, which weigh 500 to 1000 g, may occasionally live as long as 100 years (and the oldest known living bird, as of 2003, was a 51-year-old Laysan Albatross; photo of a Laysan Albatross to the right was taken by Chandler Robbins). Birds achieve these remarkable life spans despite possessing several traits that modern theories of aging suggest should make them substantially shorter-lived than mammals. First, reactive oxygen species, which are highly damaging molecules produced as a normal by-product of aerobic metabolism, are now thought to contribute substantially to the generalized degenerative changes of aging across a wide spectrum of species. An index of exposure to such molecules is thought to be lifetime oxygen consumption per cell in an organism. Yet oxygen consumption per unit time per cell of birds can range as high as 2 to 2.5 times that of mammals. So when this level of oxygen consumption is combined with the long lives of birds, one may postulate that lifetime exposure to reactive oxygen species in long-lived avian cells may be 10 to 20 times that of short-lived mammals such as mice and 2 to 5 times that of even long-lived mammals such as humans. Clearly birds have evolved some type of especially effective mechanisms for protecting against the accumulation of oxidative damage. The details of these protective measures remain elusive. It may be that because of more effective electron scavenging in avian mitochondria, birds produce fewer reactive oxygen species per unit of oxygen consumption or that birds have more active enzymes for detoxifying these molecules. However, research into these areas has so far been limited (Austad 1997).

Examples of the eight main types of avian ecosystem service providers. (a) Seed disperser: Black-mandibled Toucan, Ramphastos ambiguus (Las Cruces, Costa Rica). (b) Pollinator: Snowy-bellied Hummingbird, Amazilia edward (Las Cruces, Costa Rica). (c) Nutrient depositor: Gentoo Penguin, Pygoscelis papua (Port Lockroy, Antarctica). (d) Grazer: Cackling Goose, Branta hutchinsii (California, USA). (e) Insectivore: Golden-crowned Warbler, Basileuterus culicivorus (Las Cruces, Costa Rica). (f) Raptor: Bald Eagle, Haliaeetus leucocephalus (Alaska, USA). (g) Scavenger: Andean Condor, Vultur gryphus (Patagonia, Chile). (h) Ecosystem engineer: Slaty-tailed Trogon, Trogon massena (Pipeline Road, Panama). From: Sekercioglu (2006).
The ecological functions of birds (Sekercioglu 2006) — Birds are mobile links that are crucial for maintaining ecosystem function, memory and resilience. Avian ecological functions encompass all three major linkages: genetic, resource and process. Seed-dispersing frugivores and pollinating nectarivores are genetic linkers that carry genetic material from one plant to another or to habitat that is suitable for regeneration, respectively. Piscivorous birds are resource linkers whose droppings transport aquatic nutrients to terrestrial environments. Grazers, such as geese, and predatory birds, such as insectivores and raptors are trophic process linkers that influence plant, invertebrate and vertebrate prey populations, respectively. Ecosystem engineers, such as woodpeckers are non-trophic process linkers that modify their environment by physically transforming materials from one state to another. Mobile link categories are not mutually exclusive. Birds, particularly colonial species (e.g. social weavers Philetairus socius) and woodpeckers, can modify their environment substantially by constructing nests, which are often used by a variety of other species. Thus, many bird species are both trophic and physical process linkers. Piscivorous bird colonies can carry out all of these linkages as these birds can consume fish, deposit nutrients, engineer ecosystems via burrow construction and even disperse seeds that are adhered to their feet.
Birds also benefit humans by providing important ecosystem services such as: provisioning services via game meat for food, down for garments and guano for fertilizer; regulating services by scavenging carcasses and waste, by controlling populations of invertebrate and vertebrate pests, by pollinating and dispersing the seeds of plants; cultural services, as exemplified by the prominent roles of birds in art and religion and by the billions of dollars spent on birdwatching; and supporting services by cycling nutrients and by contributing to soil formation (Sekercioglu 2006).
Lecture Notes:
Literature Cited
Alonso, P. D., A. C. Milner, R. A. Ketcham, M. J. Cookson & T. B. Rowe. 2004. The avian nature of the brain and inner ear of Archaeopteryx. Nature 430: 666 – 669.
- Asara, J. M., M. H. Schweitzer, L. M. Freimark, M. Phillips, and L. C. Cantley. 2007. Protein Sequences from Mastodon and Tyrannosaurus Rex Revealed by Mass Spectrometry. Science 316: 280-285.
Austad, S. N. 1997. Birds as models of aging in biomedical research. ILAR Journal 38.
Bokma, F. 2002. A statistical test of unbiased evolution of body size in birds. Evolution 56: 2499-2504.
Bokma, F. 2004. Why most birds are small – a macro-ecological approach to the evolution of avian body size. Ph.D. dissertation, University of Oulu, Oulu, Finlan
Bormashenko, E., Y. Bormashenko, T. Stein, and G. Whyman. 2007. Why do pigeon feathers repel water? Hydrophobicity of pennae, Cassie-Baxter wetting hypothesis and Cassie-Wenzel capillary-induced wetting transition. Journal of Colloid and Interface Science 311: 212-216.
Carney, R. M., J. Vinther, M. D. Shawkey, L. D’Alba, and J. Ackermann. 2012. New evidence on the colour and nature of the isolated Archaeopteryx feather. Nature Communications 3:637.
Choiniere, J. N., X. Xu, J. M. Clark, C. A. Forster, Y. Guo, and F. Han. 2010. A basal Alvarezsauroid theropod from the Early Late Jurassic of Xinjiang, China. Science 327: 571-574.
Clark, J. and K. Middleton. 2006. Bird evolution. Current Biology 16: R350-R354.
Codd, J. R., D. F. Boggs, S. F. Perry, and D. R. Carrier. 2005. Activity of three muscles associated with the uncinate processes of the giant Canada Goose Branta canadensis maximus. Journal of Experimental Biology 208: 849 -857.
Codd, J. R., P. L. Manning, M. A. Norell, and S. F. Perry. 2007. Avian-like breathing mechanics in maniraptoran dinosaurs. Proceedings of the Royal Society of London B, online early.
Davis, P. G. 1998. The bioerosion of bird bones. Int. J. Osteoarcheology 7:388-401.
Degernes, L. A. and A. Feduccia. 2001. Tenectomy of the supracoracoideus muscle to deflight Pigeons (Columba livia) and Cockatiels (Nymphicus hollandicus). Journal of Avian Medicine and Surgery 15: 10–16.
Dove, C. J., A. M. Rijke, X. Wang, and L. S. Andrews. 2007. Infrared analysis of contour feathers: the conservation of body heat radiation in birds. Journal of Thermal Biology 32: 42-46.
Fajardo, R. J., E. Hernandez, and P. M. O’Connor. 2007. Postcranial skeletal pneumaticity: a case study in the use of quantitative microCT to assess vertebral structure in birds. Journal of Anatomy 211: 138-147.
Feduccia, A. 2005. Mesozoic aviary takes form. Proceedings of the National Academy of Science USA 102: 18998-19002.
Feduccia, A. and J. Nowicki. 2002. The hand of birds revealed by early ostrich embryos. Naturwissenschaften 89: 391–393.
Gatesy, S. M. and K. P. Dial. 1996. From frond to fan: Archaeopteryx and the evolution of short-tailed birds. Evolution 50: 2037-2048.
Geist, N. R. and A. Feduccia. 2000. Gravity-defying behaviors: identifying models for protoaves. American Zoologist 40: 664-675.
Galis, F., M. Kundrát, and B. Sinervo. 2002. An old controversy solved: bird embryos have five fingers. Trends in Ecology and Evolution 18:7-9.
Glen, C. L. and M. B. Bennett. 2007. Foraging modes of Mesozoic birds and non-avian theropods. Current Biology 17: R911-R912.
Guglielmo1, C. G., T. Piersma, and T. D. Williams. 2001. A sport-physiological perspective on bird migration: evidence for flight-induced muscle damage. Journal of Experimental Biology 204: 2683-2690.
Heilmann, G. 1926. The origin of birds. Witherby, London.
Huxley, T. H. 1868. On the animals which are most nearly intermediate between the birds and reptiles. Ann. Mag. Nat. Hist. 2:66–75.
Ji, Q., M. A. Norell, K.-Q. Gao, S.-A. Ji, and D. Ren. 2001. The distribution of integumentary structures in a feathered dinosaur. Nature 410: 1084-1088.
Kovacs, C. E. and R. A. Meyers. 2000. Anatomy and histochemistry of flight muscles in a wing-propelled diving bird, the Atlantic Puffin, Fratercula arctica. Journal of Morphology 244: 109-125.
Larsson, H.C.E. and G.P. Wagner. 2002. Pentadactyl ground state of the avian wing. J. Exp. Zool. (Mol. Dev. Evol.) 294: 146–151.
Linn, K. A., A. S. Templer, J. R. Paul-Murphy, R. T. O’Brien, B. K. Hartup, & J. A. Langenberg. 2003. Ultrasonographic imaging of the Sandhill Crane (Grus canadensis) intertarsal joint. Journal of Zoo and Wildlife Medicine 34: 144-152.
Louchart, A., and L. Viriot. 2011. From snout to beak: the loss of teeth in birds. Trends in Ecology and Evolution 26: 663-673.
Organ, C. L., A. M. Shedlock, A. Meade, M. Pagel, and S. V. Edwards. 2007. Origin of avian genome size and structure in non-avian dinosaurs. Nature 446: 180-184.
Ostrom, J. H. 1976. Archaeopteryx and the origin of birds. Biological Journal of the Linnean Society 8:91–182.
Padian, K. 1996. Early bird in slow motion. Nature 382:500-401.
Prum, R. O. 1999. Development and evolutionary origin of feathers: Journal of Experimental Zoology 285: 291–306.
Prum, R. O., S. Andersson, and R.H. Torres. 2003. Coherent scattering of ultraviolet light by avian feather barbs. Auk 120:163-170.
Schweitzer, M. H., Z. Suo, R. Avci, J. M. Asara, M. A. Allen, F. T. Arce, and J. R. Horner. 2007. Analyses of Soft Tissue from Tyrannosaurus rex Suggest the Presence of Protein. Science 316: 277-280.
Seebacher, F. 2003. Dinosaur body temperatures: the occurrence of endothermy and ectothermy. Paleobiology 29: 105-122.
Sekercioglu, D. H. 2006. Increasing awareness of avian ecological function. Trends in Ecology and Evolution 21: in press and online.
- Tickle, P. G., A. R. Ennos, L. E. Lennox, S. F. Perry, and J. R. Codd. 2007. Journal of Experimental Biology 210: 3955-3961.
van den Hout, P. J., T. Piersma, A. Dekinga, S. K. Lubbe, and G. H. Visser. 2006. Ruddy Turnstones Arenaria interpres rapidly build pectoral muscle after raptor scares. Journal of Avian Biology 37: 425-430.
van Oers, K., P.J. Drent, P. De Goede and A.J. van Noordwijk. 2004. Realized heritability and repeatability of risk-taking behaviour in relation to avian personalities. Proceedings of the Royal Society of London, Series B 271: 65-73.
Wagner, G. P. and J.A. Gauthier. 1999. A solution to the problem of the homology of the digits in the avian hand. Proc. Natl Acad. Sci. USA 96: 5111–5116.
Xu, X., Z. Zhou, and X. Wang. 2000. The smallest known non-avian theropod dinosaur. Nature 408: 705 – 708.
Yu, M., Z. Yue, P. Wu, D.-Y. Wu, J.-A. Mayer, M. Medina, R.B. Widelitz, T.-X. Jiang, and C.-M. Chuong. 2004. The developmental biology of feather follicles. Int. J. Dev. Biol. 48: 181-191.
Zhang, F., S. L. Kearns, P. J. Orr, M. J. Benton, Z. Zhou, D. Johnson, X. Xu, and X. Wang. 2010. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature, advanced online.
Zhang, F., Z. Zhou, X. Xu, X. Wang, and C. Sullivan. 2008. A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature 455: 1105-1108.
Zhou, Z. 2004. The origin and early evolution of birds: discoveries, disputes, and perspectives from fossil evidence. Naturwissenschaften 91: 455-471.
Zimmer, K. 1935. Beitrage zur mechanik der atmung bei den v ögeln in stand und flug. Aufgrund anatomischer-physiologisher und experimenteller Studien. Zoologica 88:1 -69.
Useful links:
Back to BIO 554/754 syllabus
Back to Avian Biology
| BIO 554/754 Ornithology Lecture Notes 2 – Bird Flight I |
 |
| Origin of FlightExactly how birds acquired the ability to fly has baffled scientists for years. Archaeopteryx provided a starting point for speculation. Built like a dinosaur, but with wings, scientists guessed at how a hypothetical ancestor might have taken flight. Some scientists support the arboreal hypothesis (e.g., Feduccia 1996) and suggest that the ancestors of Archaeopteryx lived in trees and glided into flapping flight (Figure to the right). But others argue that the claws of Archaeopteryx weren’t suited to climbing. So, others support the cursorial hypothesis (e.g., Burgers and Chiappe 1999) and suggest that these ancestors used their long, powerful legs to run fast with their arms outstretched, and were at some point lifted up by air currents and carried into flapping flight (Figure to the bottom right).Studying living animals can throw light on their evolutionary past. Ken Dial (2003) of the Flight Lab at the University of Montana noticed the ability of gamebird chicks to escape danger by scrambling up vertical surfaces. The chicks first run very fast, flapping their immature, partially feathered wings, frantically creating enough momentum to run up a vertical surface to safety. Could this survival instinct be the origin of flight?
And finally, James Carey, a UC Davis demographer and ecologist, has proposed that the evolution of bird flight is linked to parental care (Carey and Adams 2001). Whatever the origins, dinosaurs, and birds, eventually took to the air. Images & text used with permission. |
|
| Dinosaurs’ flapping led to flight? The wing-assisted incline running hypothesis — The feathered forelimbs of small, two-legged dinosaurs may have helped them run up hills or other inclines to escape predators. This half running, half flapping may have evolved into an ability to fly. Dial (2003) reported findings suggesting that the ability to fly evolved gradually. Feathers may have first protected animals from cold & wet weather, then been used out of necessity when something with big teeth was chasing them. Even before their wings develop enough to fly, some living birds use them to improve traction and gain speed. Dial studied birds, like partridges, capable of only limited flight. Energetically, “It’s a lot cheaper to run than fly,” Dial said. So these baby birds, with big feet & powerful legs, use them in combination with their wings, first to stay balanced and grounded, then to take on steeper and steeper inclines. Using this “wing assisted incline running,” Chukar Partridges can negotiate 50 degree inclines right after hatching, 60 degree slopes at 4 days old, and at 20 days, can perform a vertical ascent. “The wings help them stick to the ground,” said Dial. The wings only come into play on steep angles because at about a 50 – 60 degree incline the birds start slipping. Then they begin a head to tail movement, like a reptile, that pushes them to the ground to enhance traction. “They use their wings like spoilers on a race car, to give their feet better traction,” he said. Use of this wing-assisted running doesn’t stop when the birds are old enough to fly. Adult birds often choose the running and flapping option instead of flying because it is more energy efficient. – Written by Marsha Walton, CNN |
Chukar Partridge flapping & climbing

Jesus-Christ Hypothesis. Because all fossils of Archaeopteryx come from marine sediments, suggesting a coral-reef setting, Videler (2005)
suggests that, like the Jesus Christ lizards [Basiliscus spp.; (a)], Archaeopteryx and its ancestors were ‘Jesus-Christ dinosaurs’ running over water
to escape from predators and travel between islands in the coral lagoons of central Europe 150 million years ago. At first, both thrust and weight
support were provided by the feet slapping against the water. Later, the wings gradually took over some of the weight support, with every step
toward increased lift providing a fitness advantage.
 |
 |
Biplane wing planform and flight performance of a feathered dinosaur (Chatterjee and Templin 2007) — Microraptor gui, a four-winged dromaeosaur from the Early Cretaceous of China, provides strong evidence for an arboreal-gliding origin of avian flight. It possessed asymmetric flight feathers not only on the manus but also on the pes. A previously published reconstruction shows that the hindwing of Microraptor supported by a laterally extended leg would have formed a second pair of wings in tetrapteryx fashion. However, this wing design conflicts with known theropod limb joints that entail a parasagittal posture of the hindlimb. Here, we offer an alternative planform of the hindwing of Microraptor that is concordant with its feather orientation for producing lift and normal theropod hindlimb posture. In this reconstruction, the wings of Microraptor could have resembled a staggered biplane configuration during flight, where the forewing formed the dorsal wing and the metatarsal wing formed the ventral one. The contour feathers on the tibia were positioned posteriorly, oriented in a vertical plane for streamlining that would reduce the drag considerably. Leg feathers are present in many fossil dromaeosaurs, early birds, and living raptors, and they play an important role in flight during catching and carrying prey. A computer simulation of the flight performance of Microraptor suggests that its biplane wings were adapted for undulatory “phugoid” gliding (see below) between trees, where the horizontal feathered tail offered additional lift and stability and controlled pitch. Like the Wright 1903 Flyer, Microraptor, a gliding relative of early birds, took to the air with two sets of wings.

Phugoid gliding is a type of flight where a plane (or Microraptor gui) pitches up and climbs, and then pitches down and descends,
accompanied by speeding up and slowing down as it goes “uphill” and “downhill (Source: www.centennialofflight.gov).

A dinosaur fossil unearthed in the Gobi Desert of Mongolia shows that miniaturization, a hallmark of bird origins and a necessary precursor of flight, occurred progressively in primitive dinosaurs (Credit: F. Ippolito, American Museum of Natural History).
Theropod size and avian flight — An 80-million-year-old dinosaur fossil unearthed in the Gobi Desert of Mongolia demonstrates that miniaturization, long thought to be a hallmark of bird origins and a necessary precursor of flight, occurred progressively in primitive dinosaurs. “This study alters our understanding of the evolution of birds by suggesting that flight is a ‘spin-off’ adaptation of a much earlier trend toward miniaturization in certain dinosaur lineages,” said H. R. Lane (NSF). “Paleontologists thought that miniaturization occurred in the earliest birds, which then facilitated the origin of flight,” said Alan Turner (American Museum of Natural History). “Now the evidence shows that this decrease in body size occurred well before the origin of birds and that the dinosaur ancestors of birds were, in a sense, pre-adapted for flight.” Because most dinosaurs were too massive to fly, miniaturization is considered crucial to the origin of flight. To date, fossil evidence of miniaturization and other characteristics leading to flight has been sparse. While other dinosaurs of the Cretaceous Period were increased in size, this newly discovered dinosaur (Mahakala omnogovae) represented a step towards miniaturization necessary for flight. Other groups that evolved flight, such as pterosaurs and bats, all evolved from small ancestors. With the discovery of Mahakala, Turner et al. (2007) showed that this miniaturization occurred much earlier.” Mahakalal was nearly full-grown when it died, measuring less than two feet in length and weighing about 24 ounces. In the broader context of the dinosaur family tree, Mahakala shows that dinosaurs’ size decreased progressively as they evolved toward birds. “Many of the animals that were thought to look like giant lizards only a few years ago are now known to have been feathered, to have brooded their nests, to have been active, and to have had many other defining bird characteristics, like wishbones and three forward-facing toes,” said Mark Norell (American Museum of Natural History). “We can now add that the precursors of birds were also small, primitive members of a lineage that later grew much larger–long after their divergence from the evolutionary stem leading to birds.”

Phylogeny and body size change within paravian theropods. A temporally calibrated cladogram depicting the phylogenetic position of Mahakala and paravian body size through time and across phylogeny is shown. Silhouettes are to scale, illustrating the relative magnitude of body size differences. Left-facing silhouettes near open circles show reconstructed ancestral body sizes. Ancestral paravian body size is estimated to be 600 to 700 g and 64 to 70 cm long. The ancestral deinonychosaur, troodontid, and dromaeosaurid body size is estimated at 700 g. Large numbers (1, 2, 3, and 4) indicate the four major body increase trends in Deinonychosauria. Ma, Maastrichtian; Ca, Campanian; Sa, Santonian; Co, Coniacian; Tu, Turonian; Ce, Cenomanian; Ab, Albian; Ap, Aptian; Bar, Barremian; Hau, Hauterivian; Va, Valanginian; Ber, Berriasian; Ti, Tithonian; Ki, Kimmeridgian. Ma, million years ago (From: Turner et al. 2007).

The Berlin Archaeopteryx. In the earliest cast of the main slab (A), long hindlimb feathers are visible (B) (Longrich 2006).
 Berlin Archaeopteryx. A, Plumage of the right hindlimb. B, Schematic drawing. Abbreviations: cov, covert feathers; prt, pretibial feathers; pst, shafts of post-tibial feathers; pub, pubis; ti, tibia (Longrich 2006). |
 Berlin Archaeopteryx. A, Reconstruction. B, Life restoration. The hindlimbs have been abducted to 90° so as show the area of the leg plumage. The area of the hindlimbs was measured distal to the body contour and proximal to the ankle (Longrich 2006). |
Case closed?? Support for the arboreal hypothesis — Feathers cover the legs of the Berlin specimen of Archaeopteryx lithographica, extending from the cranial surface of the tibia and the caudal margins of both tibia and femur. These feathers exhibit features of flight feathers rather than contour feathers, including vane asymmetry, curved shafts, and a self-stabilizing overlap pattern. Many of these features facilitate lift generation in the wings and tail of birds, suggesting that the hindlimbs acted as airfoils. Longrich (2006) presented a new reconstruction of Archaeopteryx where the hindlimbs formed approximately 12% of total airfoil area. Depending upon their orientation, the hindlimbs could have reduced stall speed by up to 6% and turning radius by up to 12%. The presence of the “four-winged” planform in both Archaeopteryx and basal Dromaeosauridae indicates that their common ancestor used both forelimbs and hindlimbs to generate lift. The presence of flight feathers on the hindlimbs is inconsistent with the cursorial hypothesis, the Jesus-Christ hypothesis, and the wing-assisted incline running hypothesis; in these scenarios, such a specialization would serve no purpose, and would impede locomotion. The evidence presented by Longrich (2006), therefore, supports an arboreal origin of avian flight, and suggests that arboreal parachuting and gliding preceded the evolution of avian flight.
Evolution of flight: a summary — Although the timing remains unclear, the first step toward the evolution of flight involved a reduction in size, with their ancestors decreasing in size during the Triassic and well before the evolution of birds and flight. Endothermy must have evolved sometime between the early Late Triassic, when dinosaurs first appeared in the fossil record and the evolution of modern birds whose ancestors first appeared in the early Late Jurassic. More specifically, coelurosaurs, a diverse group of dinosaurs that likely included the ancestors of birds, exhibited substantial and sustained morphological transformation and this rapid evolution of skeletal diversity may indicate rapidly changing selection pressures as a result of radiation into new ecological niches. The evolution of endothermy may have been more likely in lineages, such as the smaller coelurosaurs, exposed to new selection pressures rather than in more conservative, larger-bodied, lineages (Schluter 2001). For example, the body temperatures of small dinosaurs (< 100 kg) that lived at mid-latitudes (45-55°) or higher would have been well below 30°C during winter if they were crocodile-like ectotherms (Seebacher 2003). Selection pressures for morphological and physiological thermoregulatory adaptations would likely have been strongest in such dinosaurs. Of course, without insulation, the thermoregulatory advantages gained from elevated resting metabolic rates would be limited. Most skin impressions from dinosaurs indicate the presence of naked skin (Sumida and Brochu 2000), except for integumentary structures in coelurosaurs that may have afforded thermal insulation (Chen et al. 1998). Although other dinosaurs may have possessed integumentary structures with insulatory qualities, current evidence suggests that these evolved only in coelurosaurs. The earliest known feathers stem from the Late Jurassic, so if those feathers possessed insulating qualities, true endothermy may have evolved sometime after that (Seebacher 2003).
By the time Archaeopteryx arrived on the scene, therefore, birds obviously had the basic features needed for flight – relatively small with feathers and, if not truly endothermic, then, at minimum, an elevated metabolism. The question then is how the ancestors of Archaeopteryx, with the necessary characteristics, first took to the air. Several hypotheses have been proposed. Primary among them are the arboreal hypothesis (e.g., Feduccia 1996), with the ancestors of Archaeopteryx living in trees (or at least climbing into trees on a regular basis) and initially gliding before developing flapping flight, and the cursorial hypothesis (e.g., Burgers and Chiappe 1999), with these ancestors using long, powerful legs to run fast with their arms (wings) outstretched and, eventually, developing sufficient lift to take flight.
Two additional hypotheses include the WAIR (wing-assisted incline running) hypothesis and the ‘Jesus-Christ’ hypothesis. Dial (2003) noticed the ability of young Chukars to escape danger by scrambling up inclined surfaces. The chicks first run very fast, flapping their rather small, partially feathered wings to creating enough momentum to run up a inclined surface to safety. The ancestors of birds may have using proto-wings in a similar fashion, with wings eventually evolving to the point of permitting not only running up inclined surfaces but, for an animal running across the ground, flight. Because all fossils of Archaeopteryx come from marine sediments, suggesting a coral-reef setting, Videler (2005) suggested that, like the Jesus Christ lizards (Basiliscus spp.), Archaeopteryx and its ancestors were ‘Jesus-Christ dinosaurs’ running over water to escape from predators and travel between islands in the coral lagoons of central Europe 150 million years ago. At first, both thrust and weight support were provided by the feet slapping against the water. Later, the wings gradually took over some of the weight support, with every step toward increased lift providing a fitness advantage.
There is currently no clear concensus in support of any of these hypotheses for the origin of bird flight. However, a four-winged dromaeosaur (Microraptor gui) from the early Cretaceous of China provides evidence for an arboreal-gliding origin of avian flight. It had asymmetric flight feathers not only on the forelimb, but on the hindlimb as well. Chatterjee and Templin (2007) proposed that the wings of Microraptor could have resembled a staggered biplane configuration during flight, where the forewing formed the dorsal wing and the hindwing formed the ventral one. The contour feathers on the tibia fo the hindlimb were positioned posteriorly, oriented in a vertical plane for streamlining that would reduce the drag considerably. Leg feathers are present in many fossil dromaeosaurs, early birds, and living raptors, and they play an important role in flight during catching and carrying prey. A computer simulation of the flight performance of Microraptor suggested that its biplane wings were adapted for undulatory “phugoid” gliding between trees, where the horizontal feathered tail offered additional lift and stability and controlled pitch. Thus, Microraptor, a gliding relative of early birds, apparently took to the air with two sets of wings.
In further support of the arboreal hypothesis, feathers also cover the legs of the Berlin specimen of Archaeopteryx lithographica, extending from the cranial surface of the tibia and the caudal margins of both tibia and femur. These feathers exhibit features of flight feathers rather than contour feathers, including vane asymmetry, curved shafts, and a self-stabilizing overlap pattern. Many of these features facilitate lift generation in the wings and tail of birds, suggesting that the hindlimbs acted as airfoils. Longrich (2006) presented a new reconstruction of Archaeopteryx where the hindlimbs formed approximately 12% of total airfoil area. Depending upon their orientation, the hindlimbs could have reduced stall speed by up to 6% and turning radius by up to 12%. The presence of “four-wings” in both Archaeopteryx and basal Dromaeosauridae suggests that their common ancestor used both forelimbs and hindlimbs to generate lift. In addition, the presence of flight feathers on the hindlimbs is inconsistent with the cursorial hypothesis and the Jesus-Christ hypothesis because flight feathers on the hindlimbs would seemingly limit running speed. The evidence presented by Longrich (2006), therefore, supports an arboreal origin of avian flight, and suggests that arboreal parachuting and gliding likely preceded the evolution of avian flight just as it apparently did in the evolution of flight in bats (Speakman 2001) and pterosaurs (Naish and Martill 2003).

Archaeopteryx (Source: Nick Longrich)
Although the presence of flight feathers on the hindlimbs would seem to support the arboreal hypothesis for the origin of flight, such feathers do not necessarily indicate that Archaeopteryx and its immediate ancestors were strictly tree-dwellers. Many present-day birds spend time both in trees and on the ground and Archaeopteryx likely did the same. With hindlimb feathers, as well as flight muscles less developed than those of present day birds, Archaeopteryx may have found it difficult, if not impossible, to take off directly from the ground. So, to take flight, Archaeopteryx and its ancestors likely sought elevated perches like trees for ‘launching.’ In doing so, they may very well have used wing-assisted incline running just like some present-day birds. For example, several petrels are known to climb trees to launch themselves into the air (del Hoyo et al. 1992), and, for some seabirds, the presence of ‘take-off trees’ is important in selection of breeding habitat (Sullivan and Wilson 2001).
Neurological evidence supports the idea that Archaeopteryx was a rather accomplished flyer. Reconstruction of the braincase and inner ear of Archaeopteryx revealed strong similarities to present-day birds, with areas of the brain involved in hearing and vision enlarged and an enlarged forebrain that would enhance the rapid integration of sensory information required in a flying animal (Alonso et al. 2004).
Flight requires lift, which occurs because wings move air downwards. Lift is created only when air strikes a wing at an angle (i.e., the angle of attack). When the leading edge of a wing is higher than the trailing edge, the bottom of the wing ‘pushes’ the air forward and creates an area of high pressure below and ahead of the wing. At the same time, air is deflected downward so, because of Newton’s Third Law of Motion (for every action there is an equal and opposite reaction), the wing is deflected upward. Both the upper and lower surfaces of the wing deflect the air. The upper surface deflects air down because the airflow “sticks” to the wing surface and follows the tilted wing (the “Coanda effect”).
Because of inertia, air moving over the top of the wing tends to keep moving in a straight line while, simultaneously, atmospheric pressure tends to force air against the top of the wing. The inertia, however, keeps the air moving over the wing from ‘pushing’ against the top of the wing with as much force as it would if the wing wasn’t moving. This creates an area of lower pressure above the wing. Because air tends to move from areas of high pressure to areas of low pressure, air tends to move from the high pressure area below and ahead of the wing to the lower pressure area above and behind the wing. This air moves, therefore, toward the trailing edge of the wing, or the same direction as the airflow created by the wing’s motion. As a result, air flows faster over the top of the wing. Because air under the wing is dragged slightly in the direction of travel, it moves slower than does the air moving over the top of the wing. Thus, air is flowing slower beneath the bottom of the wing. The faster-moving air going over the top of the wing exerts less pressure than the slower-moving air under the wing and, as a result, the wing is pushed upwards by the difference in pressure between the top and the bottom (the Bernoulli effect). So, both the development of low pressure above the wing (Bernoulli’s Principle) and the wing’s reaction to the deflected air underneath it (Newton’s third Law) contribute to the total lift force generated.

Note that air, both above AND below the wing, is deflected downward.
Source: An excellent article about lift (“Lift doesn’t suck”) by Roger Long.




Clear example of how wings deflect air downward. Notice the trough formed in the clouds.
(Source: http://www.grc.nasa.gov)
Airfoil
Airfoil and air flow

How do airfoil shape, camber, and angle of attack influence lift?
Click on this image!
When the curvature over the top becomes greater by increasing the angle of attack (below), the amount of lift generated increases because the force with which the wing is pushed upward increases. Eventually, however, if the angle of attack becomes too great, the flow separates off the wing and less lift is generated. The result is stalling. Birds also tend to stall at low speeds because slower moving air may not move smoothly over the wing.



If the angle of attack is too great, air flow over the top of the wing may become more turbulent & the result is less lift.
|
A
 |
C
 |
|
B
 |
D
 |
Angle of attack decreases with increasing speed. Angle of attack during two wingbeats of a Ringed Turtle-Dove (Streptopelia risoria) flying at 1 meter/sec (A), 5 meters/sec (B), 9 meters/sec (C), and 17 meters/sec (D). Angle of attack at low speeds peaked at 52 degrees (proximal wing) and 43 degrees (distal wing), much
greater than those commonly used by aircraft (0-15 degrees). At faster speeds, mean angle of attack decreased to 9-14 degrees (proximal wing) and -5-14 degrees
(distal wing), within the range employed by aircraft. Shaded areas indicate downstroke; solid line = distal wing & dashed line = proximal wing (Hedrick et al. 2002).
At low speeds (such as during take-off & landing), birds can maintain smooth air flow over the wing (and, therefore, maintain lift) by using the alula (also called the bastard wing). The alula is formed by feathers (usually 3 or 4) attached to the first digit.

http://en.wikipedia.org/wiki/Flight_feather
 Source: http://courses.egr.duke.edu/PROJECTS/Modules/mammals/lesson/wingskeleton.html |
 Source: http://www.windsofkansas.com/fltphoto.html |

Source: http://www.lam.mus.ca.us/birds/guide/pg018.html
 At increasing angles of attack, an eddy starts to propagate from the trailing edge towards the leading edge of the wing. As a result, air flowing over the top of the wing separates from the upper surface and lift is lost. However, when coverts are lifted upward by the eddy, they prevent the spread of the eddy and work as ‘eddy-flaps.’ Source: www.bionik.tu-berlin.de/user/giani/vortrag/ |
 The ‘covert eddy-flaps’, by preventing the spread of the eddy toward the leading edge of the wing, help maintain lift (i.e., prevent stalling) at high angles of attack, e.g., when taking off or landing. |
 Eoalulavis hoyasi. Top, fluorescence induced ultraviolet photo of the specimen before preparation. Bottom, reconstruction. A – alula, PR – primary remiges, and SR – secondary remiges (Sanz and Ortega 2002). |
The fossilized remains of a tiny bird provides evidence that birds flew as nimbly 115 million years ago as their descendants do today. The fossilized bird, Eoalulavis hoyasi, was found in a limestone quarry in Spain (Sanz et al. 1996). About the size of a goldfinch, the bird had an alula, or bastard wing, that would have helped it stay aloft at slow speeds. Eoalulavisis the most primitive bird known with an alula. Archaeopteryx probably flapped and glided, but did not have an alula. Eoalulavis provides evidence that by 30 million years after Archaeopteryx, at least one group of early birds had developed the alula. Eoalulavis hoyasi, which means “dawn bird with a bastard wing from Las Hoyas,” was discovered at a site where a freshwater lake existed millions of years ago. The bird may have hunted by wading in shallow water the way plovers and other shorebirds do today. |

Linchpin of flight
Using computer modeling, treadmills and the fossil record, researchers have shown that the acrocoracohumeral ligament (AHL), a short band of tissue
that connects the humerus to the shoulder joint in birds, was a critical element in the evolution of flight (Image: David Baier/Brown University).
To find out if this ligament played the same shoulder-stabilizing role in primitive animals, they looked to the alligator. Alligators are close relatives of birds and both are archosaurs, the “ruling reptiles” that appeared on the planet some 250 million years ago and evolved into the dinosaurs that dominated during the Mesozoic Era. So to understand the sweep of evolution, the alligator was a great starting place. In the lab, three alligators were put on motorized treadmills and X-ray videos were made. The video was used to make a 3D computer animation that showed the precise positioning of the shoulder as the animal walked. They found that alligators use muscles – not ligaments – to do the hard work of supporting the shoulder. Then Baier studied the skeleton of Archaeopteryx lithographica, and even traveled to Beijing to examine the fossilized remains of Confuciusornis, Sinornithoides youngi and Sinornithosaurus millenii, close relatives of modern birds recently discovered in China.
If the acrocoracohumeral ligament was critical to the origin of flight, Baier expected to find evidence of it in Archaeopteryx. Surprisingly, however, the new ligament-based force balance system appears to have evolved more gradually in Mesozoic fliers. “What this means is that there were refinements over time in the flight apparatus of birds,” Baier said. “Our work also suggests that when early birds flew, they balanced their shoulders differently than birds do today. And so they could have flown differently. Some scientists think they glided down from trees or flapped off the ground. Our approach of looking at this force balance system can help us test these theories.”
| Of course, a bird moving through the air is opposed by friction & this is called drag. The types of drag acting on birds are parasitic drag, pressure (or induced) drag, & friction (or profile) drag. Parasitic drag is caused by friction between a bird’s body and the air (and is termed parasitic because the body does not generate any lift). Induced drag occurs when the air flow separates from the surface of a wing, while friction drag is due to the friction between the air and bird moving through the air. Friction drag is minimized by a wing’s thin leading edge (wings ‘slice’ through the air). Induced drag occurs at low speeds and at higher speeds as, at wing tips, air moves from the area of high pressure (under the wing) to the area of low pressure (top of the wing). As wings move through the air, this curling action causes spirals (vortices) of air (see photo of continuous vortices to the right) which can disrupt the smooth flow of air over a wing (and reduce lift). |  |

A ‘smoke angel’ created after flares were released and caused by wingtip vortices (Photo source: US Air Force).
 |
Bird tails & flight — Most birds have rather short triangular tails when spread. In flight, the tail is influenced by the time-varying wake of flapping wings and the flow over the body. It is reasonable to assume that body, wings and tail morphology have evolved in concert. Modelling the interaction between the wings and tail suggests that the induced drag of the wing–tail combination is lower than that for the wings alone. A tail thus enables the bird to have wings that are optimized for cruising speed (with the tail furled to minimize drag) and, at low speeds, the spread tail reduces induced drag during manoeuvring and turning flight. Observations show that tails are maximally spread at low speeds and then become furled increasingly with increasing speed (Hedenström 2002).Figure to the left. Flow visualization around mounted wingless starling bodies using the smoke-wire technique in a wind tunnel at 9 ms−1. (a) The bird with intact tail and covert feathers; (b) tail feathers protruding beyond ventral coverts are trimmed to the same length as coverts; (c) tail feathers, ventral and dorsal covert feathers removed. The height of the wake increases from (a) to (c). The dorsal boundary layer also becomes increasingly turbulent in (b) and (c) compared with the intact tail-body configuration in (a). From: Hedenström (2002). |
 |
(A) Depictions of the vortex-ring and continuous-vortex gaits. (B) Cross-sectional view of the wing profile. Lift produced during flapping provides weight support (upward force) and thrust (horizontal force). In the vortex-ring gait, lift is produced only during the downstroke, providing positive upward force and forward thrust. In the continuous-vortex gait, lift is produced during both the upstroke and the downstroke. The downstroke produces a positive upward force and forward thrust; the upstroke produces a positive upward force and rearward thrust. Partial flexion of the wing during the upstroke reduces the magnitude of the rearward thrust to less than that of the forward thrust produced during the downstroke, providing net positive thrust per wingbeat (From Hedrick et al. 2002). |
| Birds are known to employ two different gaits in flapping flight, a vortex-ring gait in slow flight and a continuous-vortex gait in fast flight. In the vortex ring gait, the upstroke is aerodynamically passive (there is no bound circulation during this phase, and hence no trailing vortex), and the wings flex and move close to the body to minimize drag. In the continuous vortex gait (where each wingtip sheds a separate vortex trail during both the upstroke and downstroke), the wings are aerodynamically active throughout (i.e., lift is generated both during the downstroke and the upstroke), while the wings remain near-planar throughout and deform only by flexure at the wrist. Hedrick et al. (2002) studied the use of these gaits over a wide range of speeds in Cockatiels and Ringed Turtle-doves trained to fly in a wind tunnel. Despite differences in wing shape and wing loading, both species shifted from a vortex-ring to a continuous-vortex gait at a speed of 7 meters/sec. They found that the shift from a vortex-ring to a continuous-vortex gait depended on sufficient forward velocity to provide airflow over the wing during the upstroke similar to that during the downstroke. This shift in flight gait appeared to reflect the need to minimize drag and produce forward thrust in order to fly at high speed. |
 |
 |
Flow visualization images by helium-bubble multi-flash photography (top) and sketch of vortex wake (below)
as reconstructed by stereophotogrammetry, for the vortex ring gait of a slow-flying Rock Pigeon (Columba livia; left),
and for the continuous vortex gait of a European Kestrel (Falco tinnunculus; right) in cruising flight (Rayner and Gordon 1997).
The amount of drag varies with a bird’s mass (increased mass = increased friction drag), shape, & speed (increased speed = increased induced drag at the wing tips), and with a wing’s surface area & shape.

Increased streamlining (e.g., no trailing legs and extended head)
reduces drag (Pennycuick et al. 1996).
As described below, some wing shapes help to reduce induced drag. Wing shapes vary substantially among birds:


Skeletal elements of the wing of five species of birds scaled so the carpometacarpi are of equal length (Dial 1992).
 Theoretical wings that illustrate extremes of pointedness (shift in wingtip toward the leading edge) and convexity (decrease in acuteness of the wingtip). (a) rounded (low aspect ratio) and (b) pointed (high aspect ratio) wings; (c) concave and (d) convex wings (From: Lockwood et al. 1998). |
 Distribution of species in terms of wing pointedness and convexity. Each point represents one species. a. tern, b. duck, c. pigeon, d. gull, e. magpie, f. buzzard (soaring hawk), and g. sparrowhawk (accipiter) (From: Lockwood et al. 1998). |
Aspect ratio affects the relative magnitude of induced and profile drag; if mass, wing area, and other wing shape parameters remain constant, a long, thin high-aspect ratio wing reduces the cost of flight and extends range. However, high aspect ratio is not necessarily associated with high speed (favored by smaller wings). Elliptical wings (low aspect ratio) can maximize thrust from flapping, whereas as more pointed wing (high-speed) with a sharp wingtip minimizes wing weight and wing inertia. Short wings must be flapped at high frequency to provide sufficient thrust. So, relatively short, pointed wings allow rapid wing-beats with reduced inertia and that translates into greater speed (e.g., shorebirds, auks, and ducks). More rounded (convex) wings produce more lift toward the wingtip (where the wing moves faster) and are particularly effective for birds that fly at slow speeds (e.g., taking off from the ground) or need high levels of acceleration. Many small passerines often fly slowly or in ‘cluttered’ habitats, or need rapid acceleration to escape predators. The same is true for birds like accipiters and corvids (crows and jays; Lockwood et al. 1998).
A convenient way to describe the shape of a wing is by its aspect ratio – the ratio of length to width. Among bird wings, aspect ratios vary from about 1.5 to as high as about 18. Elliptical (or ‘rapid takeoff’) wings (above) have relatively low aspect ratios, while high speed wings & soaring wings have high aspect ratios.
- The long (or soaring) wings of birds with very high aspect ratios, like albatrosses, generate lots of lift, while the narrow, pointed shape helps reduce drag while gliding (because the small area of the pointed tip minimizes pressure differences and, therefore, turbulence at the wing tip).

Laysan Albatross wing (Source: http://www.ups.edu/biology/museum/wingphotos.html)
Masked Booby in flight
| Whistle through the wing – Birds molt for a variety of reasons. Molting regulates body temperature, keeps feathers neat and waterproof and allows seasonal changes in appearance for mating or migration. However, generating new feathers uses extra energy; staying warm with less plumage uses extra energy, and flying with smaller, work-in-progress wings requires extra energy. So, not all birds molt in the same way. Ducks, swans and geese, for example, shed all their flight feathers at once and are flightless until replacements have grown. Most other birds, however, lose and renew their feathers according to a continuous, pre-programmed sequence. This sequential molting gives rise to a range of temporary feather gaps that seem to reduce take-off speed, take-off angle and level flight speed and to impede predator evasion by raising a bird’s minimum turning radius. Anders Hedenstrom and Shigeru Sunada of Cambridge University estimated how the aerodynamics of flight are affected by molting (Hedenstrom & Sunada 1999). They estimated drag and lift by analyzing the fluid dynamics of symmetrical gaps in flat, rectangular model wings of various width-to-length (aspect) ratios, at a fixed angle with respect to air flow – a system that reasonably approximates a bird in gliding, but not flapping, flight. Although the effects were small, Hedenstrom and Sunada concluded that both feather gap size and position affect flight performance. Large gaps, and gaps in the middle of the wing impede aerodynamic efficiency more than small, wing-tip gaps. They also found that the detrimental effect of molt gaps increases with increasing aspect ratio. In other words a bird with short, broad wings, like a vulture, won’t miss a few feathers as much as one with long, narrow wings, like an albatross. “This is of great ecological significance,” they muse, “as it could help explain why large birds show relatively slow rates of molting that are associated with rather small gaps.” — Sara Abdulla, Nature Science Update | Wilson’s Storm Petrel Photo by Brian Patteson http://www.patteson.com/image2.htm |
- High speed wings, like those of falcons, swallows, & swifts, have relatively high aspect ratios. These narrow, tapering wings can be flapped rapidly to generate lots of speed with minimal drag (because, again, the small area of the pointed tip minimizes pressure differences and turbulence at the wing tip).

Chimney Swift wing (Source: http://www.ups.edu/biology/museum/wingphotos.html)
Falcons in flight!

Check this video (click on the falcon).
Gyrfalcon
Peregrine Falcon
Peregrine Falcon with a camera mounted on its back!
| How pigeons give falcons the slip— A Peregrine Falcon dive-bombing at several hundred miles an hour to knock a pigeon out of the sky would seem to be a study in single-mindedness. At those speeds, attention must be paid. But even a falcon in hot pursuit can become distracted. And what distracts it, is a patch of white feathers on the rump of an otherwise blue-gray pigeon. “The brain can be primed by a conspicuous thing,” said Alberto Palleroni. The falcon, he said, sees the conspicuous thing — the white patch — and doesn’t notice the pigeon starting to turn away and escape. “In effect, it’s a kind of a card trick or a ruse” on the part of the pigeon, Palleroni said.Palleroni et al. (2005) observed more than 1,800 falcon attacks on wild pigeons over seven years. They recorded the plumage types among the pigeons and noticed that while birds with white rump patches made up 20% of the pigeon population, very few were captured by the falcons. When a Peregrine Falcon attacks a pigeon, it plunges at speeds greater than 200 miles an hour, levels off and comes upon the pigeon from behind, punching it with what amounts to a closed fist. At those speeds even a grazing blow kills the pigeon; the falcon then circles back and picks it up. The only way the slower-flying pigeon can escape is by dipping a wing, rolling and veering off. If the falcon is distracted by the white patch, it won’t notice the dipping of the wing (which, being blue-gray, blends with the landscape) until it’s too late.Plumage color in pigeons is an independently heritable trait, Palleroni said, meaning it is not tied to selection involving sexual or other traits. So it is highly likely that the white rump feathers are an anti-predator adaptation to high-speed attacks. Not bad for a bird that many people disdain. “The feral pigeon is an amazing balance of adaptations and success,” Palleroni said. – Henry Fountain, New York Times |   Source: www.rpra.org/img/falcon-pigeon.jpg |
- High-lift wings have lower aspect ratios & there are spaces between the feathers at the end of the wing. These ‘slots’ help reduce drag at slow speeds because the separated tip feathers (shown in the White Stork pictured below) act as ‘winglets’ and spread vorticity both horizontally and vertically.
Golden Eagle in flight
Philippine Eagle (currently critically endangered)
- Wings with low aspect ratios (elliptical wings), like those of many songbirds, woodpeckers, pheasants, & quail, permit sharp turns while flying among trees & shrubs.

Spruce Grouse wing (Source: http://www.ups.edu/biology/museum/wingphotos.html)
Another important factor that influences a bird’s flying ability is wing loading – the weight (or mass) of a bird divided by wing area (grams/total wing area in square centimeters). Birds with low wing loading need less power to sustain flight. Birds considered to be the ‘best’ flyers, such as swallows & swifts, have lower wing loading values than other birds.
|
|
|
The Flight Strategy of Magnificent Frigatebirds — Frigatebirds cannot land on the sea because their feathers are not waterproof. If they did land, they would find it even harder to take off again because their legs are too short. Despite this, frigatebirds are perfectly  suited for an aerial life over the sea because they have the lowest wing-loading (large wing area & low body mass) of any bird. Weimerskirch et al. (2003) investigated the movements of Magnificent Frigatebirds (Fregata magnificens) while foraging at sea off the coast of French Guiana. Because they are very light in comparison to their wing surface, frigatebirds can glide at altitudes of up to 2,500 meters. Then they glide downward, taking advantage of the next current. This flight strategy, which limits the bird’s physical efforts, is the same as that used by migratory birds during long flights over land. Migratory birds, however, avoid flying over the sea due to a lack of thermals, while frigatebirds fly over the sea. As it turns out, ascending air currents are found over the sea only in tropical regions where the waters are warm enough to create such currents on a continuous basis. Frigatebirds can therefore fly night and day using this technique. To investigate the movements of Magnificent Frigatebirds, Weimerskirch et al. (2003) fitted the birds with satellite transmitters and altimeters, which allowed them to observe that the birds only occasionally come close to the sea surface to catch prey. They catch flying fish or squid driven above the surface by underwater predators like schools of tuna or dolphins. To identify such feeding opportunities, which are very rare, requires long hours of flight at high altitudes. Frigatebirds rarely feed their young, which consequently grow very slowly. The species is, however, well-adapted: it has a low reproductive rate and parent birds care for their young for over one year, the longest period of parental care of any bird. suited for an aerial life over the sea because they have the lowest wing-loading (large wing area & low body mass) of any bird. Weimerskirch et al. (2003) investigated the movements of Magnificent Frigatebirds (Fregata magnificens) while foraging at sea off the coast of French Guiana. Because they are very light in comparison to their wing surface, frigatebirds can glide at altitudes of up to 2,500 meters. Then they glide downward, taking advantage of the next current. This flight strategy, which limits the bird’s physical efforts, is the same as that used by migratory birds during long flights over land. Migratory birds, however, avoid flying over the sea due to a lack of thermals, while frigatebirds fly over the sea. As it turns out, ascending air currents are found over the sea only in tropical regions where the waters are warm enough to create such currents on a continuous basis. Frigatebirds can therefore fly night and day using this technique. To investigate the movements of Magnificent Frigatebirds, Weimerskirch et al. (2003) fitted the birds with satellite transmitters and altimeters, which allowed them to observe that the birds only occasionally come close to the sea surface to catch prey. They catch flying fish or squid driven above the surface by underwater predators like schools of tuna or dolphins. To identify such feeding opportunities, which are very rare, requires long hours of flight at high altitudes. Frigatebirds rarely feed their young, which consequently grow very slowly. The species is, however, well-adapted: it has a low reproductive rate and parent birds care for their young for over one year, the longest period of parental care of any bird. |
Check this short video of soaring frigatebirds.
Magnificent Frigatebird

Aspect ratio vs. wing loading index in some birds, airplanes, a hang-glider, a butterfly, and a maple seed.
The numbers after various flying objects refer to aspect ratio. Fm, Fregata magnificens (Magnificent Frigatebird); Ga, Gallirallus australis (Common name: Weka;
an endemic New Zealand bird in the rail family) (From: Norberg 2002).
Different combinations of wing loading and aspect ratio permit particular flight modes and foraging strategies. Species with long wings and high aspect ratios also have low wing loadings, particularly those with low body mass, and their flight is inexpensive, e.g., many seabirds, swifts, and swallows. Birds with high wing loading and short wings, but still with high aspect ratios, are adapted to fast and rather inexpensive flight (short wings reduce profile power that is large in fast flight), e.g., loons, mergansers, geese, swans, ducks, and auks. Birds flying close to or among vegetation, e.g., flycatchers, tend to have low aspect ratios that contribute to high induced drag, but their low mass and wing loading reduce flight costs. The very low aspect ratios of many smaller birds that occupy densely-vegetated habitats, e.g., gallinaceous birds, mean that the energetic cost of flight is expensive, so these species spend much of their time walking. Birds with higher wing loading, e.g., penguins, are flightless (Norberg 2002).
Flight styles — Based on differences in aspect ratios and wing loading (Rayner 1988; see figure below), flight styles can also be categorized as either specialized or non-specialized. The non-specialists have average aspect ratios and average wing loading and are excellent flyers (capable of long flights and with good maneuverability) that typically use flapping flight. The non-specialists can be further subdivided, based on aspect ratio and speed, as slow non-specialists and fast non-specialists. In the slow category would be most passerines (Passeriformes), pelicans (Pelicaniformes), herons, egrets, ibises, and storks (Ciconiiformes), pigeons and doves (Columbiformes), cuckoos (Cuculiformes), most owls (Strigiformes), trogons (Trogoniformes), most birds in the order Gruiformes (e.g., gallinules, rails, and bustards), mousebirds (Coliiformes), woodpeckers (Piciformes), and parrots (Psittaciformes). Fast non-specialists include many falcons (Falconidae), gulls (Larinae), and storm-petrels (Hydrobatidae).
Birds with morphological attributes (aspect ratio and wing loading) that differ (beyond one or two standard deviations) from those of ‘typical’ birds exhibit specialized flight styles (Rayner 1988). Among these specialized styles are:
- (1) Marine soarers are birds that fly for long periods over the open ocean and have very high aspect-ratio wings and average or low wing loading that reduce the energetic cost of flight. Birds in this category include the albatrosses (Procellariiformes).
- (2) Divers/swimmers are birds with medium to high aspect ratios and high wing loading, including murres, loons, grebes, scoters, mergansers, ducks, and swans. These birds fly rapidly, but with limited maneuverability, characteristics useful for birds that often fly long distances (e.g., during migration or to feeding areas) and take-off and land on water where precise maneuverability is not as important.
- (3) Aerial hunters are birds with high aspect-ratio wings and low wing loading, a combination permitting rapid flight and excellent maneuverability. Aerial hunters include swallows and martins (Passeriformes), swifts (Apodiformes), nightjars (Caprimulgiformes), Swallow-tailed Kites (Falconiformes), frigatebirds (Fregatidae), terns (Sterninae), some falcons (e.g., hobbies and Eleonora’s Falcon), and tropicbirds (Phaethontidae).
- (4) Soarers/coursers include birds with low aspect ratios and low wing loading, characteristics that allow relatively large birds to either soar or fly just above the vegetation in open habitats in search of prey. Birds in the soaring category include hawks and eagles (Falconiformes), vultures, condors, and storks (Ciconiiformes), and cranes (Gruiformes). Coursing birds include some owls (e.g., Barn Owl and Short-eared Owl; Strigiformes) and harriers (Falconiformes).
- (5) Short-burst fliers are birds with low aspect ratios and high wing loading that fly infrequently and only for short distances. Birds in this category include those in the orders Galliformes (e.g., turkeys, pheasants, quail, grouse, and megapodes) and Tinamiformes (tinamous).
- (6) Hoverers are birds capable of flying in one position without wind and have high aspect ratios and, surprisingly, high wing loading. The high aspect ratio reduces the energetic cost of flight, whereas the high wing loading permits relatively fast, agile flight (Rayner 1998). The only true hoverers are the hummingbirds (Apodiformes).

Approximate centroids of major groups of birds relative to aspect ratios and wing loading (From: Rayner 1988).
|
Bird Flight Speeds (m/s) Plotted in Relation to Body Mass (kg) and Wing Loading (N/m2) for 138 Species of Six Main Monophyletic Group |
Bird flight speeds — Alerstam et al. (2007) examined the cruising speeds of 138 different species of migrating birds in flapping flight using tracking radar. Mean airspeeds among the 138 species ranged between 8 and 23 m/s (or about 18 to 51 mph). Birds of prey, songbirds, swifts, gulls, terns, and herons had flight speeds in the lower part of this range, while pigeons, some of the waders, divers, swans, geese, and ducks were fast flyers in the range 15–20 m/s (33 – 45 mph). Cormorants, cranes, and skuas were among the species flying at intermediary speeds, about 15 m/s. The diving ducks reached the fastest mean speeds, with several species exceeding 20 m/s (and up to 23 m/s). An important factor in explaining variation in flight speed was phylogenetic group; species of the same group tended to fly at similar characteristic speeds.

Explanation of the Variation in Mean Flight Speeds (Ue; m/s) among Bird Species by Different Combinations of Variables and Factors
Depending on their ecological life style and foraging, birds are adapted to different aspects of flight performance, e.g., speed, agility, lift generation, escape, take-off, and energetic cost of flight.. These adaptations are likely to have implications for the flight apparatus (anatomy, physiology, and muscle operation) and the flight behavior that may constrain the cruising flight speed. Species flying at comparatively slow cruising speeds frequently use thermal soaring (raptors and storks), are adapted for hunting and load carrying (raptors), or for take-off and landing in dense vegetation (herons). Associated with these flight habits they have a lower ratio of elevator (supracoracoideus) to depressor (pectoralis) flight muscle (particularly low among birds of prey) compared with shorebirds and waterfowl. Alerstam et al. (2007) suggested that functional differences in flight apparatus and musculature among birds of different life and flight styles (differences often associated with evolutionary origin) have a significant influence on a birds performance and speed in sustained cruising flight.
 Altitude vs. time showing rapid descents during migratory flights as recorded by radar. (A) Barn Swallow, (B) Yellow Wagtail, (C) Reed Warbler, (D) Yellow Wagtail, (E) Meadow Pipit, and (F) Yellow Wagtail. |
Diving speeds — Hedenstrom and Liechti (2001) used radar to track the flights of migrating birds as they descended from their cruising altitudes after crossing the Mediterranean Sea. Dive angles were as great as 83.5 degrees and the maximum speed recorded was 53.7 meters/sec (or about 120 miles/hour). Larger birds can attain even greater speeds, with estimates of the top speed of Peregrine Falcons as high as 89 – 157 meters/sec (or about 200-350 miles/hour). Although such estimates may be correct, their accuracy is unknown because the speed of a diving falcon is difficult to measure. The required instrumention is complex, and the dive is a brief, rare event that takes place at unpredictable places and times (Tucker 1998). |
The high wing loading of birds like grebes, loons (check Looney Lift-Off), and swans (see Tundra Swan below) means that it’s more difficult for them to generate sufficient lift to take-off. That’s why these birds often run along the surface of a lake for some distance before taking flight. They must generate enough speed to generate enough lift to get their relatively heavy bodies into the air!

Source: http://www.hedweb.com/animimag/swantako.htm
Canada Geese taking off (slow motion)
Swans taking off
 |
Take-off! — Initiating flight is challenging, and considerable effort has focused on understanding the energetics and aerodynamics of take-off for both machines and animals. Available evidence suggests that birds maximize their initial flight velocity using leg thrust rather than wing flapping (e.g., see the drawings of a European Starling taking off from the ground below). The smallest birds, hummingbirds, are unique in their ability to perform sustained hovering but have small hindlimbs that could hinder generation of high leg thrust. During take-off by hummingbirds, Tobalske et al. (2004) measured hindlimb forces on a perch mounted with strain gauges and filmed wingbeat kinematics with high-speed video. Whereas other birds obtain 80–90% of their initial flight velocity using leg thrust, the leg contribution in hummingbirds was 59% during normal take-off. Unlike other species, hummingbirds beat their wings several times as they thrust using their hindlimbs. In a phylogenetic context, these results show that reduced body and hindlimb size in hummingbirds limits their peak acceleration during leg thrust and, ultimately, their take-off velocity. Previously, the influence of motivational state on take-off flight performance has not been investigated for any bird. Tobalske et al. (2004) studied the full range of motivational states by testing performance as the birds took off: (1) to initiate flight autonomously, (2) to escape a startling stimulus or (3) to aggressively chase a conspecific away from a feeder. Motivation affected performance. Escape and aggressive take-off featured decreased hindlimb contribution (46% and 47%, respectively) and increased flight velocity. When escaping, hummingbirds shortened their body movement prior to onset of leg thrust and began beating their wings earlier and at higher frequency. Thus, hummingbirds are capable of modulating their leg and wingbeat kinetics to increase take-off velocity. |

European Starling taking off from the ground. Time notations (milliseconds) are relative to the defined start of take-off (vertical
force > 105% of body weight). Key events: wings begin unfolding (73 ms) and start of downstroke (108 ms). From: Earls (2000).
European Starling taking off in slow motion
| Landing – Birds must usually be much more precise when landing than an airplane pilot; often landing on a branch rather than a runway. During landing, birds increase the angle of attack of their wings until they stall. This decreases both speed and lift. Birds also spread and lower their tails, with the tail increasing drag & acting like a brake. Finally, legs and feet are extended for landing. Click on the Raven to the right for a cool animation . . . . (Hint: After viewing the animation, left click & hold the round cursor at the bottom; you can move it and examine more carefully what’s happening during landing). Also, check this slow-motion video of a pigeon landing on a branch and this one of a Barn Owl landing. |  |

Tree Swallow landing
Photo by Anupam Pal & used with his permission

Click on the photo to see a short video of a
Rock Pigeon landing in slow motion.
Bald Eagle landing
Eagle Owl landing
Leading-edge vortex lifts swifts — How do birds fly up to a branch and land smoothly and precisely? It turns out that they may use a completely different kind of lift — which not only works at slow speeds, but even helps birds brake to a stop. Using a model of a bird wing in water containing particles lit with a laser, Videler et al. (2004) discovered how Common Swifts (Apus apus)create lift with a “leading-edge vortex” (LEV). Think of an LEV as a horizontal tornado that forms above a sharp, swept-back wing as it cuts through the air. The vortex is a low-pressure zone. Like the low-pressure zone formed above conventional wings, it creates lift. Until this study, it had been seen in insects, but not birds. The sharp leading edges of a swift’s wings may create swirls of air (gray cones) that produce extra lift (check this Quicktime video). |
 Swifts hunt in the air, catching flying insects on the wing. To snag its prey, a swift has to be able to fly fast and make very tight turns, just like a jet fighter (From: Müller and Lentink 2004).  When gliding, a Common Swift shows a torpedo-shaped body. Its arm-wing (close to its body) has a rounded leading edge. The bird’s long, slender hand-wing has a much sharper profile. The inset shows the feathers at the hand-wing’s leading edge. |
Literature cited:
Alerstam, T., M. Rosén, J. Bäckman, P. G. P. Ericson, and O. Hellgren. 2007. Flight speeds among bird species: allometric and phylogenetic effects. PLoS Biology 5: e197.
Alonso, P. D., A. C. Milner, R. A. Ketcham, M. J. Cookson and T. B. Rowe. 2004. The avian nature of the brain and inner ear of Archaeopteryx. Nature 430: 666 – 669.
Baier, D. B., S. M. Gatesy, and F. A. Jenkins. 2006. A critical ligamentous mechanism in the evolution of avian flight. Science online publication 17 December 2006.
Burgers, P. and L. M. Chiappe. 1999. The wings of Archaeopteryx as a primary thrust generator. Nature 399: 60-62.
Carey, J.R. and J. Adams. 2001. The preadaptive role of parental care in the evolution of avian flight. Archaeopteryx 19: 97 – 108.
Chatterjee, S. and R. J. Templin. 2007. Biplane wing planform and flight performance of the feathered dinosaur Microraptor gui. Proceedings of the National Academy of Science, online early – Jan. 2007.
Chen P., Z. Dong, and S. Zhen. 1998. An exceptionally well-preserved theropod dinosaur from the Yixian Formation of China. Nature. 391:147–152.
del Hoyo, J., A. Elliott, and J. Sargatal (eds.). 1992. Handbook of birds of the world, volume 1. Lynx Edicions, Barcelona, Spain.
Dial, K. P. 1992. Avian forelimb muscles and nonsteady flight: can birds fly without using the muscles in their wings? Auk 109: 874-885.
Dial, K. P. 2003. Wing-assisted incline running and the evolution of flight. Science 299:402-404.
Earls, K. D. 2000. Kinematics and mechanics of ground take-off in the starling (Sturnus vulgaris) and the quail (Coturnix coturnix). Journal of Experimental Biology 203:725-739.
Feduccia, A. 1996. The origin and evolution of birds. Yale Univ. Press, New Haven.
Hedenström, A. 2002. Aerodynamics, evolution and ecology of avian flight. Trends in Ecology and Evolution 17: 415-422.
Hedenström, A. and F. Liechti. 2001. Field estimates of body drag coefficient on the basis of dives in passerine birds. Journal of Experimental Biology 204: 1167-1175.
Hedenstrom, A. and S. Sunada. 1999. On the aerodynamics of moult gaps in birds. Journal of Experimental Biology 202:67-76.
Hedrick, T. L., B. W. Tobalske, and A. A. Biewener. 2002.Estimates of circulation and gait change based on a three-dimensional kinematic analysis of flight in cockatiels (Nymphicus hollandicus) and Ringed Turtle-doves (Streptopelia risoria). Journal of Experimental Biology 205:1389-1409.
Lockwood, R., J. P. Swaddle, and J. M. V. Rayner. 1998. Avian wingtip shape reconsidered: wingtip shape indices and morphological adaptations to migration. Journal of Avian Biology 29: 273-292.
Longrich, N. 2006. Structure and function of hindlimb feathers in Archaeopteryx lithographica. Paleobiology 32: 417-431.
Müller, U. K. and David Lentink. 2004. Turning on a dime. Science 306: 1899 – 1900.
Naish, D. and D. M. Martill. 2003. Pterosaurs – a successful invasion of prehistoric skies. Biologist 50: 213-216.
Noberg, U. M. L. 2002. Structure, form, and function of flight in engineering and the living world. Journal of Morphology 252:52-81.
Palleroni, A., C. T. Miller, M. Hauser, and P. Marler. 2005. Predation: Prey plumage adaptation against falcon attack. Nature 434:973-974.
Pennycuick C. J., M. Klaassen, A. Kvist, and A. Lindström. 1996. Wingbeat frequency and the body drag anomaly: wind-tunnel observations on a thrush nightingale (Luscinia luscinia) and a teal (Anas crecca). Journal of Experimental Biology 199: 2757–2765.
Rayner, J. M. V. 1988. Form and function in avian flight. Current Ornithology 5: 1-66.
Sanz, J. L., L. M. Chiappe, P. Perez-Moreno, A. D. Buscalioni, J. J. Moratalla, F. Ortega, & F. J. Payata-Ariza. 1996. An Early Cretaceous bird from Spain and its implications for the evolution of avian flight. Nature 382: 442-445.
Sanz, J. L. and F. Ortega. 2002. The birds from Las Hoyas. Science Progress 85:113-130.
Schluter D. 2001. Ecology and the origin of species. Trends in Ecology and Evolution. 16:372–380.
Seebacher, F. 2003. Dinosaur body temperatures: the occurrence of endothermy and ectothermy. Paleobiology 29: 105-122.
Speakman, J. R. 2001. The evolution of flight and echolocation in bats: another leap in the dark. Mammal Review 31: 111-130.
Sullivan, W. and K.-J. Wilson. 2001. Differences in habitat selection between Chatham Petrels (Pterodroma axillaris) and Broad-billed Prions (Pachyptila vittata): implications for management of burrow competition. New Zealand Journal of Ecology 25: 65-69.
Sumida S. S., and C. A. Brochu. 2000. Phylogenetic context for the origin of feathers. American Zoologist. 40:486–503.
Tobalske, B. W., D. L. Altshuler,and D. R. Powers. 2004. Take-off mechanics in hummingbirds (Trochilidae). Journal of Experimental Biology 207: 1345-1352.
Tucker, V. A. 1998. Gliding flight: speed and acceleration of ideal falcons during diving and pull out. Journal of Experimental Biology 201: 403-414.
Turner, A. H., D. Pol,J. A. Clarke,G. M. Erickson, and M. A. Norell. 2007. A basal dromaeosaurid and size evolution preceding avian flight. Science 317: 1378-1381.
Videler, J. J. 2005. Avian flight. Oxford University Press, Oxford, UK.
Videler, J.J., E.J. Stamhuis, and G.D.E. Povel. 2004. Leading-edge vortex lifts swifts. Science 306:1960-1962.
Weimerskirch, H., O. Chastel, C. Barbraud, and O. Tostain. 2003. Frigatebirds ride high on thermals. Nature 421:333-334.
Lecture Notes:
Useful links:
Spread wing-tips of the birds as a model for drag reduction
Back to BIO 554/754 syllabus
Back to Avian Biology
| BIO 554/754 Ornithology Lecture Notes 3 – Bird Flight II |
 |
Birds fly in a variety of ways, ranging from gliding to soaring to flapping flight to hovering. Of these, the simplest type of flight is gliding.
- A gliding bird uses its weight (mass) to overcome air resistance to its forward motion. To do this effectively, of course, requires a certain mass &, as a result, only large birds, such as vultures, glide on a regular basis. When gliding, a bird loses altitude at some ‘sinking speed’ (Vs) while traveling forward at some ‘flight speed’ (V). A bird’s glide ratio equals V/Vs (the distance traveled forward per unit of altitude lost). Some of the best ‘bird gliders’ (such as Black Vultures) may travel up to 20 meters for every meter of altitude lost (or, a glide ratio of 20) (Check this short video of a Bald Eagle gliding).
- A soaring bird (e.g., Turkey Vultures) maintains or increases its altitude without flapping its wings (Check this short video of a soaring vulture). One way to do this is to take advantage of rising air, e.g.,
- updrafts are generated when a steady wind strikes a hill, cliff, or building, & this is referred to as obstruction lift:

-
- thermals, or updrafts caused by the uneven heating of air near the earth’s surface. Air over fields heats faster than air over a forest or lake. The warmer air over a field is lighter than the surrounding cooler air &, therefore, rises. However, at high altitudes the warm air begins to cool & sink. As a result, birds using thermals for lift typically fly in circles (to stay in the area of rising air; check this short video).

Source: Ákos et al. (2008)

Trajectory or flight path of a Peregrine Falcon superimposed on a black and white satellite map of the area (southeast Hungary).
Color indicates vertical velocity, with more reddish color indicating climbing within thermals and bluish color indicating sinking
(i.e., periods of gliding between thermals) (Source: Ákos et al. 2008).


Dorsal wing profile in silhouette of Argentavis is compared for scaling with those of a Bald Eagle.
Argentavis magnificens from the upper Miocene(6 million years ago) of Argentina, with an estimated mass of 70–72 kgand a wingspan of 7 m, was the world’s largest known flyingbird. Because thefossils of Argentavis are found in the foothills of the Andesto the pampas, it is likely that it used primarilyslope soaring over the windward slopes of the Andes and thermalsoaring over the open pampas. In slope soaring, a bird fliesin a region of rising air caused by upward deflection of windover a ridge or a cliff. If the sinking speed of the animalis less than the velocity of the rising air, the bird is ableto remain airborne indefinitely without flapping its wings. Cranial morphology indicates that Argentavis, like other teratorns,was an active predator rather than a scavenger.It was probably a diurnal predator, dependent on thermals forflight activity for much of the time much as large, broad-wingedcarnivorous birds we see today. Strong thermals occur by mid-dayand disappear in the evening, so thermal soaring for Argentaviswould have been possible only between those times. With a skull>55 cm long and 15 cm wide, Argentavis was capable of catchingsizeable prey with its formidable beak — From: Chatterjee et al. (2007).
- Over the open ocean, large birds like the Wandering Albatross shown below take advantage of wind velocity gradients in a type of soaring called dynamic soaring.

Wandering Albatross
© Paul Ward and Cool Antarctica
Soaring albatross
 |
Dynamic soaring — Albatrosses perform a fascinating and complicated flight maneuver called dynamic soaring, in which energy can be extracted from horizontally moving air and transferred to the bird so that an energy gain is achieved which enables it to fly continuously without flapping. Dynamic soaring is possible when the wind speed changes with altitude. This type of wind, which is called shear flow, exists in the boundary layer above the ocean surface in areas in which albatrosses are found. Dynamic soaring consists of periodically repeated cycles, with one cycle illustrated to the left: 1 – climb (windward flight); 2 – upper curve (change of flight direction to leeward); 3 – descent (leeward flight); & 4 – lower curve (change of flight direction to windward) (Sachs 2005). |

Dynamic soaring is energetically efficient. The heart rate of a Wandering Albatross was recorded over a two-day period
and its heart rate was just above resting rates when soaring, suggesting that dynamic soaring requires little more energy than
resting on land (Weimerskirch et al. 2000).
|
Procellariiform birds: the ‘tube knows’ air speed? — Albatrosses and smaller Procellariiform birds like petrels and shearwaters can travel long distances over the ocean by dynamic soaring. This method of soaring requires that these birds be able to detect variation in wind speed at various distances above the ocean surface. Pennycuick (2002) proposed that albatrosses and their relatives can use their tubenoses as a pitot tube to very accurately determine air speed. Pitot tubes on airplanes have two holes, one pointing forward (in the direction of the plane is flying) to measure what is called the stagnation (or pitot) pressure, and a side hole that measures static pressure (the ambient pressure of the surrounding air). The difference between the stagnation pressure and static pressure is called the dynamic pressure, which can be used to determine a plane’s airspeed. For example, in the diagram below, as a plane increases its airspeed, the pressure generated at the stagnation point will increase relative to the static pressure and the fluid in the differential manometer will be forced downward, out of the manometer, and upward in the tube leading out of the manometer. Pitot tubes are calibrated so that dynamic pressure readings are ‘translated’ into airspeed readings.
The tubenoses of albatrosses and other Procellariiform birds resemble pitot tubes and may function in the same way. The forward-pointing nostrils or tubenoses (pointing in the direction the birds fly), that could measure stagnation or pitot pressure, lead into nasal chambers also connected to the mouth, or oral, cavity, where pressure would correspond to static pressure. Mangold (1946) identified an expandable pocket or capsule on either side of the nasal septum of petrels and proposed that these were sense organs that could measure dynamic pressure, the difference between stagnation and static pressure, and provide information about airspeed. Additional study is needed to test Pennycuick’s (2002) and Mangold’s (1946) hypothesis.
|
- Of course, most birds flap their wings when they fly. Flapping flight involves up-and-down movement of the wings and,
 during such flight, different parts of a wing have different functions:
during such flight, different parts of a wing have different functions:
- the proximal part of the wing (basically the half closest to the body) moves less & provides most of the lift
- the distal part of the wing moves through a wide arc and generates the thrust that propels a bird forward.
- During the downstroke (power stroke), a wing moves downward & forward. As a result, the leading edge of the wing, particularly for the distal portion of the wing, is lower than the trailing edge. As a result, as shown in the figure below, the resultant force (R) is angled forward, producing forward thrust (check out canaries flying in slow motion or a Bald Eagle taking flight!).
- During the upstroke (recovery stroke), the tips of the primaries separate (look at the Canada Goose image below) & these ‘slots’ allow passage of air through them (which reduces friction as the wing comes up). Also, the wing is partially folded at the wrist & elbow and drawn in toward the body to reduce drag.


Different forces along a flapping wing. (A) There is little vertical movement of the wing close to the bird’s body, but
the distal portion of the wing is angled downward (with the leading edge lower than the trailing edge) and air moving past the distal wing
is moving faster, and at a different angle, because of the wing’s flapping motion. (B) At cross-section X, the lift is almost vertical. (C) At
cross-section Y, because of the angle, the lift force is tilted forward and produces forward thrust. D = drag force, L = lift force, R = resultant force
(From: Alexander 2002; Fig. 4.6).

These images, taken from a high-speed recording of a cockatiel flying at 1 meter/sec, show the tip-reversal upstroke.
In the first frame, the wing has already reversed direction and the humerus has been elevated. In the second frame,
the primary feathers have rotated slightly to create gaps between successive feathers. Between the second and third frames,
the rotated primaries sweep upward as the wrist joint extends. By the third frame, the primaries have been rotated back into
their standard orientation and the wing has begun to move forward as well as upward (Hedrick et al. 2004).
<br> <br>
A Black-billed Magpie flying in a windtunnel at two different speeds
wearing custom respirometry masks.
Cockatiel flying in a wind tunnel
Gull in slow motion
Birds taking off – slow motion
Gulls in slow motion
Most species of birds do not flap their wings continuously during flight. Rather, they exhibit one of two intermittent flight patterns: flap-gliding and flap-bounding. Mathematical models predict that flap-bounding is energetically cheaper than continuous flapping flight at high speeds, while flap-gliding is more efficient than continuous flapping at low speeds. However, few species of bird exhibit both types of intermittent flight, so flap-bounding may be a compromise between the need to maintain muscle contractions at an optimal velocity and the need to vary power output and flight speed. In addition, the primary flight muscle, the pectoralis, of many small birds is composed of a single muscle fiber type, further limiting the range of useful strain rates for these species. Thus, a “fixed-gear hypothesis” suggests that the only economical method for small birds to vary power output is to intermittently bound. However, investigators at the Flight Laboratory at the University of Montana have found that some small birds, such as Budgerigars and European Starlings, do exhibit both types of intermittent flight, with flap-gliding being used at lower speeds, and flap-bounding at higher speeds. This suggests that some small birds are capable of optimizing their flight styles despite the theoretical constraints of their muscle composition.


Flap-gliding flight path (left) and flap-bounding flight path (right)
Source: http://www.biology.leeds.ac.uk/staff/jmvr/Flight/PWV/index1.htm
Flap-gliding European Sparrowhawk (Accipiter nisus)

Timing of a flap-bounding cycle relative to wing movements, altitude, and body angle (relative to horizontal) for a
Zebra Finch flying in a wind tunnel (Videler 2005, based on Tobalske et al. 1999).
Dark-eyed Junco – flap-bounding flight
Source: http://biology.umt.edu/flightlab/Intermittent.htm
As flight speed increased in a wind tunnel, budgerigars that exhibited intermittent flight at all speeds tended to flex their
wings during intermittent non-flapping periods, apparently in response to increased profile drag (Tobalske and Dial 1994).

Bounding flight is not seen in birds of greater than 300 grams, thus likely to be constrained by size. The small birds which most frequently utilize bounding tend to have short rounded wings (low aspect ratio), poorly suited for gliding, making undulating flight less aerodynamically attractive.
(Source: www.biology.leeds.ac.uk/staff/jmvr/Flight/PWV/index1.htm)
- A few birds using hovering flight. Some birds, like American Kestrels, ‘hover’ or remain in place by flying into the wind at a speed equal to that of the wind, and other birds, like Ospreys (to the right) hover momentarily while foraging. But hummingbirds (check this short video) are able to remain in the same place in still air as long as they wish — they are true hoverers. A hovering hummer keeps its body at about a 45 degree angle to the ground and moves its wings in more or less a figure-eight pattern, with the “eight” lying on its side. Hummingbirds, unlike other birds, can also fly backwards (video of a hummingbird in slow-motion).

Source: http://www.njsouth.com/leamingsrun.htm

Click on this photo to see a hummingbird in slow motion.
Female Ruby-throated Hummingbird (about 45 wingbeats/second)
Female Ruby-throated Hummingbird (slow motion)
Broad-tailed Hummingbirds (slow motion)

Source: http://www.ae.utexas.edu/design/humm_mav/theory.html
|
||
Does a hummingbird fly like an insect or a bird? A bit like both. Many experts had argued that hummingbirds’ skill at hovering, of which insects are the undisputed masters, means that the two groups may stay aloft in the same way: by generating lift from a wing’s upstroke as well as the down. This turns out to be only partially true. Other birds get all of their lift from the downstroke (during slow flight and when hovering; not during faster flight), and insects manage to get equal lift from both up and down beats (check this short video), but hummingbirds lie somewhere in between and gett about 75% of their lift from the downstroke and 25% from the upstroke.  Credit: Nicolle Rager Fuller, NSF Insects attain the same lift with both strokes because their wings actually turn inside out. A hummingbird, with wings of bone and feathers, isn’t quite so flexible. But the birds are still very efficient. “Their wings are a marvellous result of the considerable demands imposed by sustained hovering flight,” Warrick says. “Provided with enough food, they can hover indefinitely.” The researchers add that the hummingbird’s flapping bears a striking resemblance to that of large insects such as hawkmoths, an example of how evolution can produce similar engineering solutions in hugely distant animal groups. – Michael Hopkin, Nature News |

In contrast to other birds, the hummingbird wing is free to rotate in all directions at the shoulder
because it’s a ball-and-socket joint (unique to hummingbirds and swifts).
(Source: http://www.ae.utexas.edu/design/humm_mav/theory.html)
Read more at: http://phys.org/news/2012-07-pair-uncover-secret-hummingbirds-ability.html#jCp
Read more at: http://phys.org/news/2012-07-pair-uncover-secret-hummingbirds-ability.html#jCp
Hummingbirds flying in the rain — Flight in rain represents a greater challenge for smaller animals because the relative effects of water loading and drop impact are greater at reduced scales given the increased ratios of surface area to mass. Nevertheless, it is well known that small volant taxa such as hummingbirds can continue foraging even in extreme precipitation. Ortega-Jimenez and Dudley (2012) evaluated the effect of four rain intensities (i.e. zero, light, moderate and heavy) on the hovering performance of Anna’s Hummingbirds (Calypte anna) under laboratory conditions. Light-to-moderate rain had only a marginal effect on flight kinematics; wingbeat frequency of individuals in moderate rain was reduced by 7% relative to control conditions. By contrast, birds hovering in heavy rain adopted more horizontal body and tail positions, a position that may reduce the number of drops hitting a bird’s wings and keep it more stable in the air. In heavy rain, hummingbirds also increased wingbeat frequency substantially, while reducing stroke amplitude when compared with control conditions. The ratio between peak forces produced by single drops on a wing and on a solid surface suggests that feathers can absorb associated impact forces by up to approximately 50%. Remarkably, hummingbirds hovered well even under heavy precipitation (i.e., 270 mm h−1) with no apparent loss of control, although mechanical power output assuming perfect and zero storage of elastic energy was estimated to be about 9% and 57%t higher, respectively, compared with normal hovering.
Links:
How hummingbirds weather the storm
Secret of hummingbirds’ ability to fly in the rain
| Hovering is hard work for most birds – Ever seen a songbird hover over a crowded feeding station, waiting for a perch to open up so it can land and eat? Looks like hard work, doesn’t it? It is, which is why hovering is something most birds don’t like to do — or can’t do — for very long. Dial et al. (1997) surgically implanted strain gauges in the wings of three Black-billed Magpies. The devices measured the force exerted by the main flapping muscle with each wing beat. The birds then flew in a wind tunnel at a range of speeds. The strain gauge allowed the scientists to calculate the power (the amount of work done per unit time) required to maintain a given speed. Hovering took nearly twice as much power as flying at average speed, the researchers found. Even when the magpies flew at top speed, they expended far less power than they did when they hovered. Evidence suggested that when they hovered, the birds were working at their physical limits. Their wing muscles appeared to be employing anaerobic metabolism, a source of energy that can’t be sustained for long. There are clearly exceptions to this. Hummingbirds, the authors note, have an unusual shoulder design that allows them to generate lift on both down-beat and up-beat. But birds with a body design similar to magpies are likely to have strict limits on their abilities to fly standing still. |
Kestrel hovering
White-tailed Kite hovering
Formation Flying
Some birds, like geese & cranes, are often observed flying in V-formation. The reason is wingtip vortices. The birds take advantage of the upwind side of the vortex shedding off the bird in front of them. This updraft actually lifts the bird up, making the flight a little easier.
 |
 |

A vortex is formed in the wake of each wingtip, creating downflow behind the wing and uplift outside
the wake, as indicated at the tip of the right wing of the right-hand bird. A trailing bird can take energetic advantage
of this uplift by flying at a suitably lateral position relative to the bird ahead. Theory suggests that the optimal wingtip
overlap for the trailing bird is about one tenth of the wingspan b. A distance of about 0.78b separates the centers
of the two trailing vortices from a bird or aircraft (Andersson and Wallander 2004).

See “Mystery of bird ‘V’ formation solved” (BBC News)
Winged Migration
Flying in a flock comes at a cost — Flying birds often form flocks, with social, navigational, and anti-predator implications. Further, flying in a flock can result in aerodynamic benefits, thus reducing power requirements, as demonstrated by a reduction in heart rate and wingbeat frequency in pelicans flying in a V-formation. But how general is an aerodynamic power reduction due to group-flight? V-formation flocks are limited to moderately steady flight in relatively large birds, and may represent a special case. What are the aerodynamic consequences of flying in the more usual ‘cluster’ flock? Usherwood et al. (2011) used data from back-mounted Global Positioning System (GPS) and inertial sensors to show that pigeons (1) maintain powered, banked turns like aircraft, imposing dorsal accelerations of up to 2 g, effectively doubling body weight and quadrupling induced power requirements, (2) increase flap frequency with increases in all conventional aerodynamic power requirements, and (3) increase flap frequency when flying near, particularly behind, other birds. Therefore, unlike V-formation pelicans, pigeons do not gain an aerodynamic advantage from flying in a flock. Indeed, the increased flap frequency, whether due to direct aerodynamic interactions or requirements for increased stability or control, suggests a considerable energetic cost to flight in a tight cluster.
Food and formation help birds fly efficiently. Swimming after a heavy meal may not be wise – but flying is another matter. Birds fly more efficiently when loaded with food, recent research suggests, helping to explain how they can migrate thousands of kilometres without stopping (Kvist et al. 2001). And a second study has confirmed the century-old suspicion that birds fly in a V formation to save substantial amounts of energy (Weimerskirch et al. 2001). Anders Kvist at Lund University in Sweden and his colleagues looked at flying efficiency in Red Knots (shown to the right), small waders that double in size for their annual migration from Siberia to Africa. Fully fed, Red Knots flying in a wind tunnel for 6-10 hours extracted significantly more power from each unit of food. This might help to explain why birds often make long non-stop flights even when they don’t have to cross an ocean or desert, says Kvist. “Since efficiency increases when the birds are heavy, it might not be as bad to make long flights as people thought.” The research flies in the face of computer predictions that birds are less efficient when full. Says bird aerodynamics specialist Jeremy Rayner of the University of Leeds: “It’s a major advance, because it has disproved something we’ve held on to for a long time.” The finding is “extremely unexpected”, agrees John Speakman who works on animal energy use at the University of Aberdeen. “This changes our whole view of migrational strategies in terms of how much fat birds should deposit to cross, say, the Sahara Desert.” Understanding the relationship between food and flight might help ecologists to measure the impact of habitat change on migratory birds, Speakman says. “If you’re deciding whether to flood an estuary, for example, this could help you make more sensible predictions about how it will affect birds that use the estuary as a stopover.” It is unclear how birds increase their efficiency when migrating, Kvist says. Puzzlingly, they don’t adopt the most economical strategy at all times. Kvist speculates that when birds are breeding they may keep reserves of strength for sudden manoeuvres such as speeding up or swerving to avoid a predator. amounts of energy (Weimerskirch et al. 2001). Anders Kvist at Lund University in Sweden and his colleagues looked at flying efficiency in Red Knots (shown to the right), small waders that double in size for their annual migration from Siberia to Africa. Fully fed, Red Knots flying in a wind tunnel for 6-10 hours extracted significantly more power from each unit of food. This might help to explain why birds often make long non-stop flights even when they don’t have to cross an ocean or desert, says Kvist. “Since efficiency increases when the birds are heavy, it might not be as bad to make long flights as people thought.” The research flies in the face of computer predictions that birds are less efficient when full. Says bird aerodynamics specialist Jeremy Rayner of the University of Leeds: “It’s a major advance, because it has disproved something we’ve held on to for a long time.” The finding is “extremely unexpected”, agrees John Speakman who works on animal energy use at the University of Aberdeen. “This changes our whole view of migrational strategies in terms of how much fat birds should deposit to cross, say, the Sahara Desert.” Understanding the relationship between food and flight might help ecologists to measure the impact of habitat change on migratory birds, Speakman says. “If you’re deciding whether to flood an estuary, for example, this could help you make more sensible predictions about how it will affect birds that use the estuary as a stopover.” It is unclear how birds increase their efficiency when migrating, Kvist says. Puzzlingly, they don’t adopt the most economical strategy at all times. Kvist speculates that when birds are breeding they may keep reserves of strength for sudden manoeuvres such as speeding up or swerving to avoid a predator.Birds also conserve fuel by flying in V formations. By measuring heart rates, researchers in France now have proof that pelicans use 11-14% less energy flying together, even when they are not perfectly positioned to take advantage of the wake from those in front of them. Configured flight may create a stream of air that allows birds to glide longer, suggests Henri Weimerskirch, the biologist at the National Centre of Scientific Research at Villiers en Bois, who led the study. “If you look closely, you see that the birds at the back are gliding more than the leader.” People have been asking whether V formations are more efficient for more than 100 years, Speakman says, but no one had measured energy savings before. “They took a century-old problem and went to the heart of it,” he says. —- Written by Erica Klarreich. |
Flight Metabolism
All birds have high metabolic rates, and flying birds have even higher rates. The metabolic cost of flight depends on the type of flight (gliding, soaring, flapping, or hovering), wing shape, and speed. Of course, flapping flight and hovering are the most costly types of flight. Laboratory studies of birds trained to fly in wind tunnels (like the one below) indicate that the metabolic ‘cost’ of flapping flight can be anywhere from about 7 to 15 times a bird’s basal metabolic rate.

Source: http://www.swe.org/iac/LP/wind_03.html
Cockatiel in a wind tunnel


Total mechanical (aerodynamic; Paero) power output during flapping flight
at different flight speeds in Cockatiels (Nymphicus hollandicus). Data represents means ± SE.
Pind = induced power, Ppar = parasite power, and Ppro = profile (friction) power (Brighton 2007).

Superficial (left) and deep (right) flight muscles of a Black-billed Magpie (Tobalske et al. 1997).

Distribution of energy during flight (Bejan 2005).
|
A 10,000 km non-stop flight — Four Bar-tailed Godwits (Limosa lapponica) flew their way into the record books with nonstop flights of more than 10,000 km from New Zealand to the Yellow Sea. The godwits, tracked by satellite transmitters in March 2007, did not stop to eat or drink on the first leg of their northern migration that ends in Alaska in May. Phil Battley, an ecologist at Massey University, said it had been suspected that the birds could fly such distances but now it had been proved. No other animal has shown such endurance, he said. The female godwits took 6 to 7 days to cover the route, flying at altitudes up to 2 km and at an average speed of 56 km/h. When the godwits leave New Zealand, they are clinically obese, but lose about half their body weight during each portion of their migratory flight. After arriving in the tidal flats of the Yellow Sea, off China and South Korea, they stay for a month or two to refuel. Battley said “It’s the equivalent of riding the Tour de France but keeping it up for a week nonstop.” |
|
Birds, of course, get around in ways other than flying. In fact, some birds are flightless and depend entirely on walking, running, or 
swimming to get from place to place. Some birds spend most of their time on or in water. Birds have special adaptations of the legs, feet, & wings for terrestrial and aquatic (swimming and diving) locomotion.
- Walking, running, hopping, & waddling – birds that travel along the ground regularly often have relatively long legs. Among the ratites, such as Ostriches (check this video!) and Emus (or check this video), there has been a reduction in the number of toes (less weight at end of the limb = more efficient locomotion).

With short, light tails (due to reduction in the number of caudal vertebrae) and large flight muscles, natural selection has favored
positioning of bird feet under a more cranial positioned center of mass. This is achieved by a subhorizontal orientation of the femur
and, when walking and running, the knee acting as the main fulcrum near the bird’s center of mass (From: Nyakatura et al. 2012).
Western Grebes
Running shorebirds
Wood Ducks walking
Waddling makes most of penguin’s short legs – It may not be graceful, but the penguin’s waddle makes perfect sense to scientists, who found that the bird’s side-to-side gait conserves energy. University of California researchers found that the gait works like a pendulum, with energy stored at the end of each swing for the bird’s next step. “Our findings indicate that walking is expensive for penguins not because of their waddling, but because they have such short legs that require their leg muscles to generate force very quickly when they walk,” said Timothy Griffin, a UC Berkeley graduate student. Griffin and Kram (2000) decided to study penguins because they seem to be doing everything wrong. An earlier study showed penguins were burning twice as many calories when walking as other animals of similar size. But researchers found the problem was the penguins’ legs, not their jerky side-to-side movements. The Emperor penguins studied at San Diego’s Sea World, for instance, were at least 3 feet tall but had legs only about 10 inches long. Penguins burn about the same amount of calories as animals with similar leg lengths, Griffin said. The researchers coaxed penguins across a force platform — “kind of a fancy bathroom scale,” says Griffin — with bits of fish. Using scale measurements and videos, the scientists measured the side-to-side and fore-and-aft forces the penguins exert while walking, as well as the vertical forces supporting their weight. The five penguins studied had a walking speed of about 1.5 feet per second. The percentage of energy retained during two steps is called the recovery rate. Humans have a recovery rate of about 65 percent. The penguins studied by Griffin and Kram had an impressive recovery rate of up to 80 percent.

Head movements in walking Whooping Cranes. (A) One frame of a video recording of a walking crane, showing method
of measurement of head, body, and leg position. The head is fit with a graphical model of the eye and bill, the body with a circle scaled to
head and leg size and centered over the pelvis of the bird, and each lower leg with a line segment extending from the ankle to the foot
(green, right leg; red, left leg). (B) One sequence of measurements, at intervals of 33 ms, of a spontaneously foraging Whooping Crane
through several complete stepping cycles. The bird walked at an average speed of about 0.46ms–1. During this sequence, the right
foot completed nearly 3 steps and the left foot, about 2.5 steps. The head was stabilized throughout most of each foot’s step, with its
positions at each of these times indicated by the arrows. (Watch a crane walk, click here!; video by Thomas Cronin).
Avian head bobbing — Many species of birds move their heads forward through a series of successive, fixed positions when walking. This unique ‘head-bobbing’ behavior stabilizes visual fields during body movement, preventing motion blur of the retinal image. Gaze stabilization could be required for successful visual search, particularly for moving objects, but the time available for stabilization varies with walking speed. No direct evidence has been published showing that birds favor the stabilization phase while foraging either for moving or immobile food. Cronin et al. (2005) examined head-bobbing behavior in foraging Whooping Cranes (Grus americana) as they searched the ground for food, and found that they walk at speeds that allow the head to be immobilized at least 50% of the time. The stable phase of bird head-bobbing movements is particularly interesting because the behavior, unique to birds, clearly contributes to visual gaze stabilization. Pigeons head-bob when landing, and herons stabilize their heads rigidly when walking or when their perch moves, almost certainly for visual function. Head movements nevertheless play essential roles in vision, giving visual cues for distances and relative locations of objects, providing an opportunity for changes in head angle, and permitting birds to fixate new objects of visual interest.
- Climbing – birds that climb, like woodpeckers, nuthatches, Black-and-white Warblers, and Pied Monarchs have sharply recurved claws to help grip the substrate (e.g., bark of a tree)

White-breasted Nuthatch
Source: http://animalpicturesarchive.com/animal/APAsrch3.cgi?qt=nuthatch
Black-and-white Warbler

A complete ‘hop’ of a treecreeper climbing upwards on a vertical trunk. The sequence is from lower left to upper right.
Birds adapted for climbing, like woodpeckers and treecreepers, have sharply recurved claws and toes, sometimes relatively long, that can be spread apart to help firmly grip the substrate (typically tree bark). Other adaptations for climbing differ with foraging habits. Climbers that typically move up trees, like woodpeckers (Picidae) and treecreepers (Certhidae), have relatively short legs (particularly the tibotarsus) that keep their center of mass close to the substrate and a long, stiff tail that provides support against the force of gravity. As woodpeckers and treecreepers move up a tree, they ‘hop’ upward and inward (to counteract the force of gravity that tends to pull them away from a vertical tree trunk), moving both feet in unison. Tail support provides two advantages: (1) the long tail creates a long baseline between the points of attachment (feet and tail) and, the longer this baseline, the smaller the horizontal force between feet and bark against which the bird must work when pulling itself towards the trunk when hopping upward, and (2), when not moving, the tail, rather than the leg muscles, supports part or all of the bird’s weight (Norberg 1981).
Climbing birds that use their tail for support almost always move upwards when foraging. Foraging woodpeckers and treecreepers approaching the top of one tree, typically fly downward to a lower position on another tree then again climb upwards and, when approaching the top, repeat the process. Not only does such a foraging strategy make sense energetically (because flying downward is less costly), but attempting to move downward when foraging would create at least three problems (Norberg 1981): (1) difficulty in seeing where to grasp the bark after a hop, (2) the stiff tail could get caught on the irregular surface of the bark, and (3) potential prey would be alerted to the presence of a possible predator before the bird could get in a position to capture them.
Nuthatches (Sittidae) are adapted for climbing downward as well as upwards. Their relatively short tails are not used for support and, rather than hopping, nuthatches walk up and down tree trunks and branches with alternating leg movements. Nuthatches have relatively long legs (particularly the tibiotarsus), allowing a relatively long baseline between the feet and reducing the horizontal force between feet and bark and the energetic cost of locomotion (Norberg 1981).
White-throated Treecreeper (Cormobates leucophaeus)
- Swimming – aquatic birds (like Tufted Ducks) typically have:
- low specific gravity (lightweight so they are very buoyant) feathers with lots of barbules & hooklets (less permeable to water) well-developed uropygial gland (secretions help keep feathers in good condition)
- webbed feet that act like oars

Source: http://www.oaklandzoo.org/atoz/video22.html
Wood Duck
A swimming and diving Anhinga
- Diving– birds that frequently dive under water, such as grebes, cormorants, & loons, have:
- relatively high specific gravities (heavier and less buoyant)
- feet located well back on the body to permit better propulsion and maneuvering underwater and/or smaller wings that permit ‘flying’ underwater (e.g., scoters, petrels, murres, and, of course, penguins like the Adelie Penguin below)

Source: http://www.bionik.tu-berlin.de/intseit2/xs2pinfi.html

Wing bones of a Jackass Penguin (Spheniscus demersus).
For wing-propelled swimming underwater, the ‘paddle’ must be highly mobile at the shoulder
(humerus-pectoral girdle articulation), but the remaining joints need to be relatively fixed to minimize
the muscle contraction needed to maintain the proper position (Louw 1992).
Emperor Penguins

Bubbles in the wake of a Pigeon Guillemot (Cepphus columba) swimming horizontally underwater,
indicating patterns of intermittent thrust mainly on the downstroke. B) Wing positions during horizontal
swimming by a Common Murre, as drawn from films taken at 32 frames/sec. Sequence is from
left to right and top row to bottom row. Angle of attack of the wings suggests substantial lift during the upstroke
(From: Lovvorn 2001).
Rapid ascent of Emperor Penguins — To jump out of water onto sea ice, Emperor Penguins must achieve sufficient underwater speed to overcome the influence of gravity when they leave the water. The relevant combination of density and kinematic viscosity of air is much lower than for water. Injection of air into boundary layers (air lubrication, i.e., an air film separates the water from the surface of a structure or a bird thus reducing friction) has been used by engineers to speed movement of vehicles (ships, torpedoes) through sea water. Based on analysis of published and unpublished underwater film, Davenport et al. (2011) hypothesized that free-ranging Emperor Penguins employ air lubrication in achieving high, probably maximal, underwater speeds (mean = 5.3 meters/sec), prior to jumps. Penguins dive to 15 to 20 meters with air in their plumage and that compressed air is released as the birds subsequently ascend while maintaining depressed feathers. Fine bubbles emerge continuously from the entire plumage, forming a smooth layer over the body and generating bubbly wakes behind the penguins. In several hours of film of hundreds of penguins, none were seen to swim rapidly upwards without bubbly wakes. Penguins descend and swim horizontally at about 2 meters/sec. Davenport et al. (2011) hypothesized that a significant proportion of the enhanced ascent speed is due to air lubrication reducing frictional and form drag and that buoyancy forces alone cannot explain the observed speeds.

Emperor Penguins create bubble trails (image: Blue Planet, BBC)
Links:
|
Why Divers Have Small Wings — Many researchers believe that small wings reduce drag underwater and, therefore, are better suited for diving. But until recently, there was no concrete evidence for the supposed benefits of small wings. Studying the effects of wing area on diving is difficult; cross-species studies never give fair comparisons. Bridge (2004) decided to study the effect of altered wing size on Common Guillemots (Uria aalge) and Tufted Puffins (Fratercula cirrhata) during their brief molting periods.
Bridge (2004) used video cameras to film the bird’s diving activity at SeaWorld California by mounting one camera in front of the pool’s But if reduced wing areas do not improve diving ability, why has natural selection favored small, pointed wings in many aquatic birds? Apparently birds with small, pointed wings are adept at high-speed, long-distance flight, essential for rapid movement between habitats. But, small, pointed wings cannot generate lift at low speed, so rapid vertical takeoffs are impossible. This is not a big problem for most diving birds because their open aquatic habitats prevent close approach by undetected predators. In addition, when the birds slow down to land, their small wings stall easily and lose lift. Fortunately, high-speed hard landings are more acceptable on water than on land. Thus, aquatic habitats relax the constraints on the evolution of small, pointed wings. — Jane Qiu, Journal of Experimental Biology |
 wing. Approximations of the percentage of intact wing area with the wing loosely extended are listed for each molt stage (Bridge 2004). |


Great Cormorant feathers have a regular and highly waterproof central part whereas the distal region is irregular and wettable.
A ventral feather is shown (From: Grémillet et al. 2005).
Unusual feather structure of Great Cormorants — Water has very high specific heat and thermal conductivity, so that diving endotherms can potentially
lose much heat to surrounding water. To deal with this challenging environment, most warm-blooded divers have highly efficient body insulation.
Marine mammals have evolved thick skins and extensive peripheral fat layers, while diving birds have dense, highly waterproof plumage that traps an
insulating layer of air. A few diving bird species, including Anhingas and cormorants are puzzling exceptions to this pattern, having plumage that is apparently
penetrated by water during submersion. The Great Cormorant (Phalacrocorax carbo) is thought to have a wettable plumage, providing low
body insulation during foraging. Great Cormorants should thus be constrained by water temperatures, and show high energy requirements.
Surprisingly, this species has one of the widest breeding distributions of all diving birds, and does not require more food than these other species.
Grémillet et al. (2005) explored this apparent paradox by comparing the insulative properties of body plumage in four subspecies of great cormorants
ranging from tropical to polar regions. The authors found that all subspecies retained an insulating air layer in their plumage, which was, however, much
thinner than for other species of diving birds. Detailed examination of the plumage showed that each cormorant body feather has a loose,
instantaneously wet, outer section and a highly waterproof central portion. This indicates that the plumage of great cormorants is only partly wettable,
and that birds maintain a thin layer of air in their plumage. These findings suggest an unusual morphological-functional adaptation to diving which balances
the antagonist constraints of thermoregulation and buoyancy.
Double-crested Cormorant
 |
Foot-propelled locomotion — When submerged, Great crested Grebes (Podiceps cristatus) swim with synchronized foot strokes, keeping their wings closely folded against the body. During the power stroke, the feet move from a cranial and ventrolateral position to a caudal and dorsomedial position relative to the body. The mean swimming speed varied from 0.7 – 1.2 meters/sec (Johansson and Norberg 2001). Dorsal (left) and lateral (right) video frames of a diving grebe. The dorsal view was recorded after reflection from a mirror. |
Western Grebe
Next: Bird Biogeography I
Lecture Notes:
Literature Cited:
Ákos, Z., M. Nagy, and T. Vicsek. 2008. Comparing bird and human soaring strategies. Proceedings of the National Academy of Sciences USA 105: 4139-4143.
Alexander, D. E. 2002. Nature’s flyers: birds, insects, and the biomechanics of flight. Johns Hopkins University Press, Baltimore, MD.
Andersson, M. and J. Wallander. 2004. Kin selection and reciprocity in flight formation? Behavioral Ecology 15: 158-162.
Bejan, A. 2005. The constructal law of organization in nature: tree-shaped flows and body size. Journal of Experimental Biology 208: 1677- 1686.
Bridge, E. S. 2004. The effects of intense wing molt on diving in alcids and potential influences on the evolution of molt patterns. Journal of Experimental Biology 207: 3003 -3014.
Brighton, C. 2007. SYNOPSIS: Mechanical power output of Cockatiel flight in relation to flight speed. Biolog-e: The undergraduate bioscience research journal. University of Leeds. <http://www.fbs.leeds.ac.uk/students/ejournal/Biolog-e/uploads/Caroline%20Brighton_synopsis.pdf> (1 February 2008).
Chatterjee, S., R. J. Templin, and K. E. Campbell, Jr. 2007. The aerodynamics of Argentavis, the world’s largest flying bird from the Miocene of Argentina. Proceedings of the National Academy of Sciences USA 104: 12398-12403.
Cronin, T. W., M. R. Kinloch, and G. H. Olsen. 2005. Head-bobbing behavior in foraging Whooping Cranes favors visual fixation. Current Biology 15: R243-R244.
Davenport, J., R. N. Hughes, M. Shorten, and P. S. Larsen. 2011. Drag reduction by air release promotes fast ascent in jumping Emperor Penguins – a novel hypothesis. Marine Ecology Progress Series 430: 171-182.
Dial, K. P., A. A. Biewener, B. W. Tobalske, and D. R. Warrick. 1997. Mechanical power output of bird flight. Science 390:67-70.
Grémillet, D., C. Chauvin, R. P. Wilson, Y, Le Maho, and S. Wanless. 2005. Unusual feather structure allows partial plumage wettability in diving Great Cormorants Phalacrocorax carbo. Journal of Avian Biology 36: 57-63.
Griffin, T.M. and R. Kram. 2000. Penguin waddling is not wasteful. Nature 408:929.
Hedrick, T. L., J. R. Usherwood and A. A. Biewener. 2004. Wing inertia and whole-body acceleration: an analysis of instantaneous aerodynamic force production in cockatiels (Nymphicus hollandicus) flying across a range of speeds. Journal of Experimental Biology 207: 1689-1702.
Johanssen, L. C. and U. M. L. Norberg. 2001. Lift-based paddling in diving grebe. Journal of Experimental Biology 204:1687-1696.
Kvist, A., A. Lindstrom, M. Green, T. Piersma, & G. H. Visser. 2001. Carrying large fuel loads during sustained bird flight is cheaper than expected. Nature 413: 730 – 732.
Louw, G. J. 1992. Functional anatomy of the penguin flipper. Journal of the South African Veterinary Association 63: 113-120.
Lovvorn, J. R. 2001. Upstroke thrust, drag effects, and stroke-glide cycles in wing-propelled swimming by birds. American Zoologist 41: 154-165.
Mangold, O. 1946. Die nase der segelnden Vo¨gel ein Organ des Stro¨mungssinnes? Naturwissenschaften 33:19-23.
Norberg, R. A. 1981. Why foraging birds in trees should climb and hop upwards rather than downwards. Ibis 123: 281-288.
Nyakatura, J. A., E. Andrada, N. Grimm, H. Weise, and M. S. Fischer. 2012. Kinematics and center of mass mechanics during terrestrial locomotion in Northern Lapwings (Vanellus vanellus, Charadriiformes). Journal of Experimental Zoology 9999A:1–15.
Ortega-Jimenez, V. M., and R. Dudley. 2012. Flying in the rain: hovering performance of Anna’s Hummingbirds under varied precipitation. Proceedings of the Royal Society B, online early.
Pennycuick, C. J. 2002. Gust soaring as a basis for the flight of petrels and albatrosses (Procellariiformes). Avian Science 2: 1-12.
Pennycuick, C. J. 2008. Information systems for flying animals. In: Theoretical Ecology Series, vol. 5. Modelling the flying bird (C. J. Pennycuick, ed.), pp. 305-331. Elsevier Inc., Amsterdam, The Netherlands.
Sachs, G. 2005. Minimum shear wind strength required for dynamic soaring of albatrosses. Ibis 147: 1 – 10.
Tobalske, B.W. and K.P. Dial. 1994. Neuromuscular control and kinematics of intermittent flight in Budgerigars (Melopsittacus undulatus). Journal of Experimental Biology 187:1-18.
Tobalske, B. W., N. E. Olson, and K. P. Dial. 1997. Flight style of the Black-billed Magpie: variation in wing kinematics, neuromuscular control, and muscle composition. Journal of Experimental Zoology 279: 313-329.
Tobalske, B. W., W. L. Peacock, and K. P. Dial. 1999. Kinematics of flap-bounding flight in the Zebra Finch over a wide range of speeds. Journal of Experimental Biology 202: 1725-1739.
Usherwood, J. R., M. Stavrou, J. C. Lowe, K. Roskilly, and A. M. Wilson. 2011. Flying in a flock comes at a cost in pigeons. Nature 474: 494-497.
Videler, J. J. 2005. Avian flight. Oxford University Press, Oxford, UK.
Warrick, D.R., B. W. Tobalske, and D. R. Powers. 2005. Aerodynamics of the hovering hummingbird. Nature 435: 1094-1097.
Weimerskirch, H., T. Guionnet, J. Martin, S. A. Shaffer, and D. P. Costa. 2000. Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proceeding of the Royal Society of London B 267: 1869-1874.
Weimerskirch, H., J. Martin, Y. Clerquin, P. Alexandre, and S. Jiraskova. 2001. Energy saving in flight formation. Nature 413: 697 – 698.
Useful links:
The intermittent flight of Zebra Finches: Unfixed gears and body lift
“Underwater Flight” of the Penguin
Back to BIO 554/754 syllabus
| BIO 554/754 Ornithology Lecture Notes 3 – Bird Flight II |
 |
Birds fly in a variety of ways, ranging from gliding to soaring to flapping flight to hovering. Of these, the simplest type of flight is gliding.
- A gliding bird uses its weight (mass) to overcome air resistance to its forward motion. To do this effectively, of course, requires a certain mass &, as a result, only large birds, such as vultures, glide on a regular basis. When gliding, a bird loses altitude at some ‘sinking speed’ (Vs) while traveling forward at some ‘flight speed’ (V). A bird’s glide ratio equals V/Vs (the distance traveled forward per unit of altitude lost). Some of the best ‘bird gliders’ (such as Black Vultures) may travel up to 20 meters for every meter of altitude lost (or, a glide ratio of 20) (Check this short video of a Bald Eagle gliding).
- A soaring bird (e.g., Turkey Vultures) maintains or increases its altitude without flapping its wings (Check this short video of a soaring vulture). One way to do this is to take advantage of rising air, e.g.,
- updrafts are generated when a steady wind strikes a hill, cliff, or building, & this is referred to as obstruction lift:

-
- thermals, or updrafts caused by the uneven heating of air near the earth’s surface. Air over fields heats faster than air over a forest or lake. The warmer air over a field is lighter than the surrounding cooler air &, therefore, rises. However, at high altitudes the warm air begins to cool & sink. As a result, birds using thermals for lift typically fly in circles (to stay in the area of rising air; check this short video).

Source: Ákos et al. (2008)

Trajectory or flight path of a Peregrine Falcon superimposed on a black and white satellite map of the area (southeast Hungary).
Color indicates vertical velocity, with more reddish color indicating climbing within thermals and bluish color indicating sinking
(i.e., periods of gliding between thermals) (Source: Ákos et al. 2008).


Dorsal wing profile in silhouette of Argentavis is compared for scaling with those of a Bald Eagle.
Argentavis magnificens from the upper Miocene(6 million years ago) of Argentina, with an estimated mass of 70–72 kgand a wingspan of 7 m, was the world’s largest known flyingbird. Because thefossils of Argentavis are found in the foothills of the Andesto the pampas, it is likely that it used primarilyslope soaring over the windward slopes of the Andes and thermalsoaring over the open pampas. In slope soaring, a bird fliesin a region of rising air caused by upward deflection of windover a ridge or a cliff. If the sinking speed of the animalis less than the velocity of the rising air, the bird is ableto remain airborne indefinitely without flapping its wings. Cranial morphology indicates that Argentavis, like other teratorns,was an active predator rather than a scavenger.It was probably a diurnal predator, dependent on thermals forflight activity for much of the time much as large, broad-wingedcarnivorous birds we see today. Strong thermals occur by mid-dayand disappear in the evening, so thermal soaring for Argentaviswould have been possible only between those times. With a skull>55 cm long and 15 cm wide, Argentavis was capable of catchingsizeable prey with its formidable beak — From: Chatterjee et al. (2007).
- Over the open ocean, large birds like the Wandering Albatross shown below take advantage of wind velocity gradients in a type of soaring called dynamic soaring.

Wandering Albatross
© Paul Ward and Cool Antarctica
Soaring albatross
 |
Dynamic soaring — Albatrosses perform a fascinating and complicated flight maneuver called dynamic soaring, in which energy can be extracted from horizontally moving air and transferred to the bird so that an energy gain is achieved which enables it to fly continuously without flapping. Dynamic soaring is possible when the wind speed changes with altitude. This type of wind, which is called shear flow, exists in the boundary layer above the ocean surface in areas in which albatrosses are found. Dynamic soaring consists of periodically repeated cycles, with one cycle illustrated to the left: 1 – climb (windward flight); 2 – upper curve (change of flight direction to leeward); 3 – descent (leeward flight); & 4 – lower curve (change of flight direction to windward) (Sachs 2005). |

Dynamic soaring is energetically efficient. The heart rate of a Wandering Albatross was recorded over a two-day period
and its heart rate was just above resting rates when soaring, suggesting that dynamic soaring requires little more energy than
resting on land (Weimerskirch et al. 2000).
|
Procellariiform birds: the ‘tube knows’ air speed? — Albatrosses and smaller Procellariiform birds like petrels and shearwaters can travel long distances over the ocean by dynamic soaring. This method of soaring requires that these birds be able to detect variation in wind speed at various distances above the ocean surface. Pennycuick (2002) proposed that albatrosses and their relatives can use their tubenoses as a pitot tube to very accurately determine air speed. Pitot tubes on airplanes have two holes, one pointing forward (in the direction of the plane is flying) to measure what is called the stagnation (or pitot) pressure, and a side hole that measures static pressure (the ambient pressure of the surrounding air). The difference between the stagnation pressure and static pressure is called the dynamic pressure, which can be used to determine a plane’s airspeed. For example, in the diagram below, as a plane increases its airspeed, the pressure generated at the stagnation point will increase relative to the static pressure and the fluid in the differential manometer will be forced downward, out of the manometer, and upward in the tube leading out of the manometer. Pitot tubes are calibrated so that dynamic pressure readings are ‘translated’ into airspeed readings.
The tubenoses of albatrosses and other Procellariiform birds resemble pitot tubes and may function in the same way. The forward-pointing nostrils or tubenoses (pointing in the direction the birds fly), that could measure stagnation or pitot pressure, lead into nasal chambers also connected to the mouth, or oral, cavity, where pressure would correspond to static pressure. Mangold (1946) identified an expandable pocket or capsule on either side of the nasal septum of petrels and proposed that these were sense organs that could measure dynamic pressure, the difference between stagnation and static pressure, and provide information about airspeed. Additional study is needed to test Pennycuick’s (2002) and Mangold’s (1946) hypothesis.
|
- Of course, most birds flap their wings when they fly. Flapping flight involves up-and-down movement of the wings and,
 during such flight, different parts of a wing have different functions:
during such flight, different parts of a wing have different functions:
- the proximal part of the wing (basically the half closest to the body) moves less & provides most of the lift
- the distal part of the wing moves through a wide arc and generates the thrust that propels a bird forward.
- During the downstroke (power stroke), a wing moves downward & forward. As a result, the leading edge of the wing, particularly for the distal portion of the wing, is lower than the trailing edge. As a result, as shown in the figure below, the resultant force (R) is angled forward, producing forward thrust (check out canaries flying in slow motion or a Bald Eagle taking flight!).
- During the upstroke (recovery stroke), the tips of the primaries separate (look at the Canada Goose image below) & these ‘slots’ allow passage of air through them (which reduces friction as the wing comes up). Also, the wing is partially folded at the wrist & elbow and drawn in toward the body to reduce drag.


Different forces along a flapping wing. (A) There is little vertical movement of the wing close to the bird’s body, but
the distal portion of the wing is angled downward (with the leading edge lower than the trailing edge) and air moving past the distal wing
is moving faster, and at a different angle, because of the wing’s flapping motion. (B) At cross-section X, the lift is almost vertical. (C) At
cross-section Y, because of the angle, the lift force is tilted forward and produces forward thrust. D = drag force, L = lift force, R = resultant force
(From: Alexander 2002; Fig. 4.6).

These images, taken from a high-speed recording of a cockatiel flying at 1 meter/sec, show the tip-reversal upstroke.
In the first frame, the wing has already reversed direction and the humerus has been elevated. In the second frame,
the primary feathers have rotated slightly to create gaps between successive feathers. Between the second and third frames,
the rotated primaries sweep upward as the wrist joint extends. By the third frame, the primaries have been rotated back into
their standard orientation and the wing has begun to move forward as well as upward (Hedrick et al. 2004).
<br> <br>
A Black-billed Magpie flying in a windtunnel at two different speeds
wearing custom respirometry masks.
Cockatiel flying in a wind tunnel
Gull in slow motion
Birds taking off – slow motion
Gulls in slow motion
Most species of birds do not flap their wings continuously during flight. Rather, they exhibit one of two intermittent flight patterns: flap-gliding and flap-bounding. Mathematical models predict that flap-bounding is energetically cheaper than continuous flapping flight at high speeds, while flap-gliding is more efficient than continuous flapping at low speeds. However, few species of bird exhibit both types of intermittent flight, so flap-bounding may be a compromise between the need to maintain muscle contractions at an optimal velocity and the need to vary power output and flight speed. In addition, the primary flight muscle, the pectoralis, of many small birds is composed of a single muscle fiber type, further limiting the range of useful strain rates for these species. Thus, a “fixed-gear hypothesis” suggests that the only economical method for small birds to vary power output is to intermittently bound. However, investigators at the Flight Laboratory at the University of Montana have found that some small birds, such as Budgerigars and European Starlings, do exhibit both types of intermittent flight, with flap-gliding being used at lower speeds, and flap-bounding at higher speeds. This suggests that some small birds are capable of optimizing their flight styles despite the theoretical constraints of their muscle composition.


Flap-gliding flight path (left) and flap-bounding flight path (right)
Source: http://www.biology.leeds.ac.uk/staff/jmvr/Flight/PWV/index1.htm
Flap-gliding European Sparrowhawk (Accipiter nisus)

Timing of a flap-bounding cycle relative to wing movements, altitude, and body angle (relative to horizontal) for a
Zebra Finch flying in a wind tunnel (Videler 2005, based on Tobalske et al. 1999).
Dark-eyed Junco – flap-bounding flight
Source: http://biology.umt.edu/flightlab/Intermittent.htm
As flight speed increased in a wind tunnel, budgerigars that exhibited intermittent flight at all speeds tended to flex their
wings during intermittent non-flapping periods, apparently in response to increased profile drag (Tobalske and Dial 1994).

Bounding flight is not seen in birds of greater than 300 grams, thus likely to be constrained by size. The small birds which most frequently utilize bounding tend to have short rounded wings (low aspect ratio), poorly suited for gliding, making undulating flight less aerodynamically attractive.
(Source: www.biology.leeds.ac.uk/staff/jmvr/Flight/PWV/index1.htm)
- A few birds using hovering flight. Some birds, like American Kestrels, ‘hover’ or remain in place by flying into the wind at a speed equal to that of the wind, and other birds, like Ospreys (to the right) hover momentarily while foraging. But hummingbirds (check this short video) are able to remain in the same place in still air as long as they wish — they are true hoverers. A hovering hummer keeps its body at about a 45 degree angle to the ground and moves its wings in more or less a figure-eight pattern, with the “eight” lying on its side. Hummingbirds, unlike other birds, can also fly backwards (video of a hummingbird in slow-motion).

Source: http://www.njsouth.com/leamingsrun.htm

Click on this photo to see a hummingbird in slow motion.
Female Ruby-throated Hummingbird (about 45 wingbeats/second)
Female Ruby-throated Hummingbird (slow motion)
Broad-tailed Hummingbirds (slow motion)

Source: http://www.ae.utexas.edu/design/humm_mav/theory.html
|
||
Does a hummingbird fly like an insect or a bird? A bit like both. Many experts had argued that hummingbirds’ skill at hovering, of which insects are the undisputed masters, means that the two groups may stay aloft in the same way: by generating lift from a wing’s upstroke as well as the down. This turns out to be only partially true. Other birds get all of their lift from the downstroke (during slow flight and when hovering; not during faster flight), and insects manage to get equal lift from both up and down beats (check this short video), but hummingbirds lie somewhere in between and gett about 75% of their lift from the downstroke and 25% from the upstroke.  Credit: Nicolle Rager Fuller, NSF Insects attain the same lift with both strokes because their wings actually turn inside out. A hummingbird, with wings of bone and feathers, isn’t quite so flexible. But the birds are still very efficient. “Their wings are a marvellous result of the considerable demands imposed by sustained hovering flight,” Warrick says. “Provided with enough food, they can hover indefinitely.” The researchers add that the hummingbird’s flapping bears a striking resemblance to that of large insects such as hawkmoths, an example of how evolution can produce similar engineering solutions in hugely distant animal groups. – Michael Hopkin, Nature News |

In contrast to other birds, the hummingbird wing is free to rotate in all directions at the shoulder
because it’s a ball-and-socket joint (unique to hummingbirds and swifts).
(Source: http://www.ae.utexas.edu/design/humm_mav/theory.html)
Read more at: http://phys.org/news/2012-07-pair-uncover-secret-hummingbirds-ability.html#jCp
Read more at: http://phys.org/news/2012-07-pair-uncover-secret-hummingbirds-ability.html#jCp
Hummingbirds flying in the rain — Flight in rain represents a greater challenge for smaller animals because the relative effects of water loading and drop impact are greater at reduced scales given the increased ratios of surface area to mass. Nevertheless, it is well known that small volant taxa such as hummingbirds can continue foraging even in extreme precipitation. Ortega-Jimenez and Dudley (2012) evaluated the effect of four rain intensities (i.e. zero, light, moderate and heavy) on the hovering performance of Anna’s Hummingbirds (Calypte anna) under laboratory conditions. Light-to-moderate rain had only a marginal effect on flight kinematics; wingbeat frequency of individuals in moderate rain was reduced by 7% relative to control conditions. By contrast, birds hovering in heavy rain adopted more horizontal body and tail positions, a position that may reduce the number of drops hitting a bird’s wings and keep it more stable in the air. In heavy rain, hummingbirds also increased wingbeat frequency substantially, while reducing stroke amplitude when compared with control conditions. The ratio between peak forces produced by single drops on a wing and on a solid surface suggests that feathers can absorb associated impact forces by up to approximately 50%. Remarkably, hummingbirds hovered well even under heavy precipitation (i.e., 270 mm h−1) with no apparent loss of control, although mechanical power output assuming perfect and zero storage of elastic energy was estimated to be about 9% and 57%t higher, respectively, compared with normal hovering.
Links:
How hummingbirds weather the storm
Secret of hummingbirds’ ability to fly in the rain
| Hovering is hard work for most birds – Ever seen a songbird hover over a crowded feeding station, waiting for a perch to open up so it can land and eat? Looks like hard work, doesn’t it? It is, which is why hovering is something most birds don’t like to do — or can’t do — for very long. Dial et al. (1997) surgically implanted strain gauges in the wings of three Black-billed Magpies. The devices measured the force exerted by the main flapping muscle with each wing beat. The birds then flew in a wind tunnel at a range of speeds. The strain gauge allowed the scientists to calculate the power (the amount of work done per unit time) required to maintain a given speed. Hovering took nearly twice as much power as flying at average speed, the researchers found. Even when the magpies flew at top speed, they expended far less power than they did when they hovered. Evidence suggested that when they hovered, the birds were working at their physical limits. Their wing muscles appeared to be employing anaerobic metabolism, a source of energy that can’t be sustained for long. There are clearly exceptions to this. Hummingbirds, the authors note, have an unusual shoulder design that allows them to generate lift on both down-beat and up-beat. But birds with a body design similar to magpies are likely to have strict limits on their abilities to fly standing still. |
Kestrel hovering
White-tailed Kite hovering
Formation Flying
Some birds, like geese & cranes, are often observed flying in V-formation. The reason is wingtip vortices. The birds take advantage of the upwind side of the vortex shedding off the bird in front of them. This updraft actually lifts the bird up, making the flight a little easier.
 |
 |

A vortex is formed in the wake of each wingtip, creating downflow behind the wing and uplift outside
the wake, as indicated at the tip of the right wing of the right-hand bird. A trailing bird can take energetic advantage
of this uplift by flying at a suitably lateral position relative to the bird ahead. Theory suggests that the optimal wingtip
overlap for the trailing bird is about one tenth of the wingspan b. A distance of about 0.78b separates the centers
of the two trailing vortices from a bird or aircraft (Andersson and Wallander 2004).

See “Mystery of bird ‘V’ formation solved” (BBC News)
Winged Migration
Flying in a flock comes at a cost — Flying birds often form flocks, with social, navigational, and anti-predator implications. Further, flying in a flock can result in aerodynamic benefits, thus reducing power requirements, as demonstrated by a reduction in heart rate and wingbeat frequency in pelicans flying in a V-formation. But how general is an aerodynamic power reduction due to group-flight? V-formation flocks are limited to moderately steady flight in relatively large birds, and may represent a special case. What are the aerodynamic consequences of flying in the more usual ‘cluster’ flock? Usherwood et al. (2011) used data from back-mounted Global Positioning System (GPS) and inertial sensors to show that pigeons (1) maintain powered, banked turns like aircraft, imposing dorsal accelerations of up to 2 g, effectively doubling body weight and quadrupling induced power requirements, (2) increase flap frequency with increases in all conventional aerodynamic power requirements, and (3) increase flap frequency when flying near, particularly behind, other birds. Therefore, unlike V-formation pelicans, pigeons do not gain an aerodynamic advantage from flying in a flock. Indeed, the increased flap frequency, whether due to direct aerodynamic interactions or requirements for increased stability or control, suggests a considerable energetic cost to flight in a tight cluster.
Food and formation help birds fly efficiently. Swimming after a heavy meal may not be wise – but flying is another matter. Birds fly more efficiently when loaded with food, recent research suggests, helping to explain how they can migrate thousands of kilometres without stopping (Kvist et al. 2001). And a second study has confirmed the century-old suspicion that birds fly in a V formation to save substantial amounts of energy (Weimerskirch et al. 2001). Anders Kvist at Lund University in Sweden and his colleagues looked at flying efficiency in Red Knots (shown to the right), small waders that double in size for their annual migration from Siberia to Africa. Fully fed, Red Knots flying in a wind tunnel for 6-10 hours extracted significantly more power from each unit of food. This might help to explain why birds often make long non-stop flights even when they don’t have to cross an ocean or desert, says Kvist. “Since efficiency increases when the birds are heavy, it might not be as bad to make long flights as people thought.” The research flies in the face of computer predictions that birds are less efficient when full. Says bird aerodynamics specialist Jeremy Rayner of the University of Leeds: “It’s a major advance, because it has disproved something we’ve held on to for a long time.” The finding is “extremely unexpected”, agrees John Speakman who works on animal energy use at the University of Aberdeen. “This changes our whole view of migrational strategies in terms of how much fat birds should deposit to cross, say, the Sahara Desert.” Understanding the relationship between food and flight might help ecologists to measure the impact of habitat change on migratory birds, Speakman says. “If you’re deciding whether to flood an estuary, for example, this could help you make more sensible predictions about how it will affect birds that use the estuary as a stopover.” It is unclear how birds increase their efficiency when migrating, Kvist says. Puzzlingly, they don’t adopt the most economical strategy at all times. Kvist speculates that when birds are breeding they may keep reserves of strength for sudden manoeuvres such as speeding up or swerving to avoid a predator. amounts of energy (Weimerskirch et al. 2001). Anders Kvist at Lund University in Sweden and his colleagues looked at flying efficiency in Red Knots (shown to the right), small waders that double in size for their annual migration from Siberia to Africa. Fully fed, Red Knots flying in a wind tunnel for 6-10 hours extracted significantly more power from each unit of food. This might help to explain why birds often make long non-stop flights even when they don’t have to cross an ocean or desert, says Kvist. “Since efficiency increases when the birds are heavy, it might not be as bad to make long flights as people thought.” The research flies in the face of computer predictions that birds are less efficient when full. Says bird aerodynamics specialist Jeremy Rayner of the University of Leeds: “It’s a major advance, because it has disproved something we’ve held on to for a long time.” The finding is “extremely unexpected”, agrees John Speakman who works on animal energy use at the University of Aberdeen. “This changes our whole view of migrational strategies in terms of how much fat birds should deposit to cross, say, the Sahara Desert.” Understanding the relationship between food and flight might help ecologists to measure the impact of habitat change on migratory birds, Speakman says. “If you’re deciding whether to flood an estuary, for example, this could help you make more sensible predictions about how it will affect birds that use the estuary as a stopover.” It is unclear how birds increase their efficiency when migrating, Kvist says. Puzzlingly, they don’t adopt the most economical strategy at all times. Kvist speculates that when birds are breeding they may keep reserves of strength for sudden manoeuvres such as speeding up or swerving to avoid a predator.Birds also conserve fuel by flying in V formations. By measuring heart rates, researchers in France now have proof that pelicans use 11-14% less energy flying together, even when they are not perfectly positioned to take advantage of the wake from those in front of them. Configured flight may create a stream of air that allows birds to glide longer, suggests Henri Weimerskirch, the biologist at the National Centre of Scientific Research at Villiers en Bois, who led the study. “If you look closely, you see that the birds at the back are gliding more than the leader.” People have been asking whether V formations are more efficient for more than 100 years, Speakman says, but no one had measured energy savings before. “They took a century-old problem and went to the heart of it,” he says. —- Written by Erica Klarreich. |
Flight Metabolism
All birds have high metabolic rates, and flying birds have even higher rates. The metabolic cost of flight depends on the type of flight (gliding, soaring, flapping, or hovering), wing shape, and speed. Of course, flapping flight and hovering are the most costly types of flight. Laboratory studies of birds trained to fly in wind tunnels (like the one below) indicate that the metabolic ‘cost’ of flapping flight can be anywhere from about 7 to 15 times a bird’s basal metabolic rate.

Source: http://www.swe.org/iac/LP/wind_03.html
Cockatiel in a wind tunnel


Total mechanical (aerodynamic; Paero) power output during flapping flight
at different flight speeds in Cockatiels (Nymphicus hollandicus). Data represents means ± SE.
Pind = induced power, Ppar = parasite power, and Ppro = profile (friction) power (Brighton 2007).

Superficial (left) and deep (right) flight muscles of a Black-billed Magpie (Tobalske et al. 1997).

Distribution of energy during flight (Bejan 2005).
|
A 10,000 km non-stop flight — Four Bar-tailed Godwits (Limosa lapponica) flew their way into the record books with nonstop flights of more than 10,000 km from New Zealand to the Yellow Sea. The godwits, tracked by satellite transmitters in March 2007, did not stop to eat or drink on the first leg of their northern migration that ends in Alaska in May. Phil Battley, an ecologist at Massey University, said it had been suspected that the birds could fly such distances but now it had been proved. No other animal has shown such endurance, he said. The female godwits took 6 to 7 days to cover the route, flying at altitudes up to 2 km and at an average speed of 56 km/h. When the godwits leave New Zealand, they are clinically obese, but lose about half their body weight during each portion of their migratory flight. After arriving in the tidal flats of the Yellow Sea, off China and South Korea, they stay for a month or two to refuel. Battley said “It’s the equivalent of riding the Tour de France but keeping it up for a week nonstop.” |
|
Birds, of course, get around in ways other than flying. In fact, some birds are flightless and depend entirely on walking, running, or 
swimming to get from place to place. Some birds spend most of their time on or in water. Birds have special adaptations of the legs, feet, & wings for terrestrial and aquatic (swimming and diving) locomotion.
- Walking, running, hopping, & waddling – birds that travel along the ground regularly often have relatively long legs. Among the ratites, such as Ostriches (check this video!) and Emus (or check this video), there has been a reduction in the number of toes (less weight at end of the limb = more efficient locomotion).

With short, light tails (due to reduction in the number of caudal vertebrae) and large flight muscles, natural selection has favored
positioning of bird feet under a more cranial positioned center of mass. This is achieved by a subhorizontal orientation of the femur
and, when walking and running, the knee acting as the main fulcrum near the bird’s center of mass (From: Nyakatura et al. 2012).
Western Grebes
Running shorebirds
Wood Ducks walking
Waddling makes most of penguin’s short legs – It may not be graceful, but the penguin’s waddle makes perfect sense to scientists, who found that the bird’s side-to-side gait conserves energy. University of California researchers found that the gait works like a pendulum, with energy stored at the end of each swing for the bird’s next step. “Our findings indicate that walking is expensive for penguins not because of their waddling, but because they have such short legs that require their leg muscles to generate force very quickly when they walk,” said Timothy Griffin, a UC Berkeley graduate student. Griffin and Kram (2000) decided to study penguins because they seem to be doing everything wrong. An earlier study showed penguins were burning twice as many calories when walking as other animals of similar size. But researchers found the problem was the penguins’ legs, not their jerky side-to-side movements. The Emperor penguins studied at San Diego’s Sea World, for instance, were at least 3 feet tall but had legs only about 10 inches long. Penguins burn about the same amount of calories as animals with similar leg lengths, Griffin said. The researchers coaxed penguins across a force platform — “kind of a fancy bathroom scale,” says Griffin — with bits of fish. Using scale measurements and videos, the scientists measured the side-to-side and fore-and-aft forces the penguins exert while walking, as well as the vertical forces supporting their weight. The five penguins studied had a walking speed of about 1.5 feet per second. The percentage of energy retained during two steps is called the recovery rate. Humans have a recovery rate of about 65 percent. The penguins studied by Griffin and Kram had an impressive recovery rate of up to 80 percent.

Head movements in walking Whooping Cranes. (A) One frame of a video recording of a walking crane, showing method
of measurement of head, body, and leg position. The head is fit with a graphical model of the eye and bill, the body with a circle scaled to
head and leg size and centered over the pelvis of the bird, and each lower leg with a line segment extending from the ankle to the foot
(green, right leg; red, left leg). (B) One sequence of measurements, at intervals of 33 ms, of a spontaneously foraging Whooping Crane
through several complete stepping cycles. The bird walked at an average speed of about 0.46ms–1. During this sequence, the right
foot completed nearly 3 steps and the left foot, about 2.5 steps. The head was stabilized throughout most of each foot’s step, with its
positions at each of these times indicated by the arrows. (Watch a crane walk, click here!; video by Thomas Cronin).
Avian head bobbing — Many species of birds move their heads forward through a series of successive, fixed positions when walking. This unique ‘head-bobbing’ behavior stabilizes visual fields during body movement, preventing motion blur of the retinal image. Gaze stabilization could be required for successful visual search, particularly for moving objects, but the time available for stabilization varies with walking speed. No direct evidence has been published showing that birds favor the stabilization phase while foraging either for moving or immobile food. Cronin et al. (2005) examined head-bobbing behavior in foraging Whooping Cranes (Grus americana) as they searched the ground for food, and found that they walk at speeds that allow the head to be immobilized at least 50% of the time. The stable phase of bird head-bobbing movements is particularly interesting because the behavior, unique to birds, clearly contributes to visual gaze stabilization. Pigeons head-bob when landing, and herons stabilize their heads rigidly when walking or when their perch moves, almost certainly for visual function. Head movements nevertheless play essential roles in vision, giving visual cues for distances and relative locations of objects, providing an opportunity for changes in head angle, and permitting birds to fixate new objects of visual interest.
- Climbing – birds that climb, like woodpeckers, nuthatches, Black-and-white Warblers, and Pied Monarchs have sharply recurved claws to help grip the substrate (e.g., bark of a tree)

White-breasted Nuthatch
Source: http://animalpicturesarchive.com/animal/APAsrch3.cgi?qt=nuthatch
Black-and-white Warbler

A complete ‘hop’ of a treecreeper climbing upwards on a vertical trunk. The sequence is from lower left to upper right.
Birds adapted for climbing, like woodpeckers and treecreepers, have sharply recurved claws and toes, sometimes relatively long, that can be spread apart to help firmly grip the substrate (typically tree bark). Other adaptations for climbing differ with foraging habits. Climbers that typically move up trees, like woodpeckers (Picidae) and treecreepers (Certhidae), have relatively short legs (particularly the tibotarsus) that keep their center of mass close to the substrate and a long, stiff tail that provides support against the force of gravity. As woodpeckers and treecreepers move up a tree, they ‘hop’ upward and inward (to counteract the force of gravity that tends to pull them away from a vertical tree trunk), moving both feet in unison. Tail support provides two advantages: (1) the long tail creates a long baseline between the points of attachment (feet and tail) and, the longer this baseline, the smaller the horizontal force between feet and bark against which the bird must work when pulling itself towards the trunk when hopping upward, and (2), when not moving, the tail, rather than the leg muscles, supports part or all of the bird’s weight (Norberg 1981).
Climbing birds that use their tail for support almost always move upwards when foraging. Foraging woodpeckers and treecreepers approaching the top of one tree, typically fly downward to a lower position on another tree then again climb upwards and, when approaching the top, repeat the process. Not only does such a foraging strategy make sense energetically (because flying downward is less costly), but attempting to move downward when foraging would create at least three problems (Norberg 1981): (1) difficulty in seeing where to grasp the bark after a hop, (2) the stiff tail could get caught on the irregular surface of the bark, and (3) potential prey would be alerted to the presence of a possible predator before the bird could get in a position to capture them.
Nuthatches (Sittidae) are adapted for climbing downward as well as upwards. Their relatively short tails are not used for support and, rather than hopping, nuthatches walk up and down tree trunks and branches with alternating leg movements. Nuthatches have relatively long legs (particularly the tibiotarsus), allowing a relatively long baseline between the feet and reducing the horizontal force between feet and bark and the energetic cost of locomotion (Norberg 1981).
White-throated Treecreeper (Cormobates leucophaeus)
- Swimming – aquatic birds (like Tufted Ducks) typically have:
- low specific gravity (lightweight so they are very buoyant) feathers with lots of barbules & hooklets (less permeable to water) well-developed uropygial gland (secretions help keep feathers in good condition)
- webbed feet that act like oars

Source: http://www.oaklandzoo.org/atoz/video22.html
Wood Duck
A swimming and diving Anhinga
- Diving – birds that frequently dive under water, such as grebes, cormorants, & loons, have:
- relatively high specific gravities (heavier and less buoyant)
- feet located well back on the body to permit better propulsion and maneuvering underwater and/or smaller wings that permit ‘flying’ underwater (e.g., scoters, petrels, murres, and, of course, penguins like the Adelie Penguin below)

Source: http://www.bionik.tu-berlin.de/intseit2/xs2pinfi.html

Wing bones of a Jackass Penguin (Spheniscus demersus).
For wing-propelled swimming underwater, the ‘paddle’ must be highly mobile at the shoulder
(humerus-pectoral girdle articulation), but the remaining joints need to be relatively fixed to minimize
the muscle contraction needed to maintain the proper position (Louw 1992).
Emperor Penguins

Bubbles in the wake of a Pigeon Guillemot (Cepphus columba) swimming horizontally underwater,
indicating patterns of intermittent thrust mainly on the downstroke. B) Wing positions during horizontal
swimming by a Common Murre, as drawn from films taken at 32 frames/sec. Sequence is from
left to right and top row to bottom row. Angle of attack of the wings suggests substantial lift during the upstroke
(From: Lovvorn 2001).
Rapid ascent of Emperor Penguins — To jump out of water onto sea ice, Emperor Penguins must achieve sufficient underwater speed to overcome the influence of gravity when they leave the water. The relevant combination of density and kinematic viscosity of air is much lower than for water. Injection of air into boundary layers (air lubrication, i.e., an air film separates the water from the surface of a structure or a bird thus reducing friction) has been used by engineers to speed movement of vehicles (ships, torpedoes) through sea water. Based on analysis of published and unpublished underwater film, Davenport et al. (2011) hypothesized that free-ranging Emperor Penguins employ air lubrication in achieving high, probably maximal, underwater speeds (mean = 5.3 meters/sec), prior to jumps. Penguins dive to 15 to 20 meters with air in their plumage and that compressed air is released as the birds subsequently ascend while maintaining depressed feathers. Fine bubbles emerge continuously from the entire plumage, forming a smooth layer over the body and generating bubbly wakes behind the penguins. In several hours of film of hundreds of penguins, none were seen to swim rapidly upwards without bubbly wakes. Penguins descend and swim horizontally at about 2 meters/sec. Davenport et al. (2011) hypothesized that a significant proportion of the enhanced ascent speed is due to air lubrication reducing frictional and form drag and that buoyancy forces alone cannot explain the observed speeds.

Emperor Penguins create bubble trails (image: Blue Planet, BBC)
Links:
|
Why Divers Have Small Wings — Many researchers believe that small wings reduce drag underwater and, therefore, are better suited for diving. But until recently, there was no concrete evidence for the supposed benefits of small wings. Studying the effects of wing area on diving is difficult; cross-species studies never give fair comparisons. Bridge (2004) decided to study the effect of altered wing size on Common Guillemots (Uria aalge) and Tufted Puffins (Fratercula cirrhata) during their brief molting periods.
Bridge (2004) used video cameras to film the bird’s diving activity at SeaWorld California by mounting one camera in front of the pool’s But if reduced wing areas do not improve diving ability, why has natural selection favored small, pointed wings in many aquatic birds? Apparently birds with small, pointed wings are adept at high-speed, long-distance flight, essential for rapid movement between habitats. But, small, pointed wings cannot generate lift at low speed, so rapid vertical takeoffs are impossible. This is not a big problem for most diving birds because their open aquatic habitats prevent close approach by undetected predators. In addition, when the birds slow down to land, their small wings stall easily and lose lift. Fortunately, high-speed hard landings are more acceptable on water than on land. Thus, aquatic habitats relax the constraints on the evolution of small, pointed wings. — Jane Qiu, Journal of Experimental Biology |
 wing. Approximations of the percentage of intact wing area with the wing loosely extended are listed for each molt stage (Bridge 2004). |


Great Cormorant feathers have a regular and highly waterproof central part whereas the distal region is irregular and wettable.
A ventral feather is shown (From: Grémillet et al. 2005).
Unusual feather structure of Great Cormorants — Water has very high specific heat and thermal conductivity, so that diving endotherms can potentially
lose much heat to surrounding water. To deal with this challenging environment, most warm-blooded divers have highly efficient body insulation.
Marine mammals have evolved thick skins and extensive peripheral fat layers, while diving birds have dense, highly waterproof plumage that traps an
insulating layer of air. A few diving bird species, including Anhingas and cormorants are puzzling exceptions to this pattern, having plumage that is apparently
penetrated by water during submersion. The Great Cormorant (Phalacrocorax carbo) is thought to have a wettable plumage, providing low
body insulation during foraging. Great Cormorants should thus be constrained by water temperatures, and show high energy requirements.
Surprisingly, this species has one of the widest breeding distributions of all diving birds, and does not require more food than these other species.
Grémillet et al. (2005) explored this apparent paradox by comparing the insulative properties of body plumage in four subspecies of great cormorants
ranging from tropical to polar regions. The authors found that all subspecies retained an insulating air layer in their plumage, which was, however, much
thinner than for other species of diving birds. Detailed examination of the plumage showed that each cormorant body feather has a loose,
instantaneously wet, outer section and a highly waterproof central portion. This indicates that the plumage of great cormorants is only partly wettable,
and that birds maintain a thin layer of air in their plumage. These findings suggest an unusual morphological-functional adaptation to diving which balances
the antagonist constraints of thermoregulation and buoyancy.
Double-crested Cormorant
 |
Foot-propelled locomotion — When submerged, Great crested Grebes (Podiceps cristatus) swim with synchronized foot strokes, keeping their wings closely folded against the body. During the power stroke, the feet move from a cranial and ventrolateral position to a caudal and dorsomedial position relative to the body. The mean swimming speed varied from 0.7 – 1.2 meters/sec (Johansson and Norberg 2001). Dorsal (left) and lateral (right) video frames of a diving grebe. The dorsal view was recorded after reflection from a mirror. |
Western Grebe
Next: Bird Biogeography I
Lecture Notes:
Literature Cited:
Ákos, Z., M. Nagy, and T. Vicsek. 2008. Comparing bird and human soaring strategies. Proceedings of the National Academy of Sciences USA 105: 4139-4143.
Alexander, D. E. 2002. Nature’s flyers: birds, insects, and the biomechanics of flight. Johns Hopkins University Press, Baltimore, MD.
Andersson, M. and J. Wallander. 2004. Kin selection and reciprocity in flight formation? Behavioral Ecology 15: 158-162.
Bejan, A. 2005. The constructal law of organization in nature: tree-shaped flows and body size. Journal of Experimental Biology 208: 1677- 1686.
Bridge, E. S. 2004. The effects of intense wing molt on diving in alcids and potential influences on the evolution of molt patterns. Journal of Experimental Biology 207: 3003 -3014.
Brighton, C. 2007. SYNOPSIS: Mechanical power output of Cockatiel flight in relation to flight speed. Biolog-e: The undergraduate bioscience research journal. University of Leeds. <http://www.fbs.leeds.ac.uk/students/ejournal/Biolog-e/uploads/Caroline%20Brighton_synopsis.pdf> (1 February 2008).
Chatterjee, S., R. J. Templin, and K. E. Campbell, Jr. 2007. The aerodynamics of Argentavis, the world’s largest flying bird from the Miocene of Argentina. Proceedings of the National Academy of Sciences USA 104: 12398-12403.
Cronin, T. W., M. R. Kinloch, and G. H. Olsen. 2005. Head-bobbing behavior in foraging Whooping Cranes favors visual fixation. Current Biology 15: R243-R244.
Davenport, J., R. N. Hughes, M. Shorten, and P. S. Larsen. 2011. Drag reduction by air release promotes fast ascent in jumping Emperor Penguins – a novel hypothesis. Marine Ecology Progress Series 430: 171-182.
Dial, K. P., A. A. Biewener, B. W. Tobalske, and D. R. Warrick. 1997. Mechanical power output of bird flight. Science 390:67-70.
Grémillet, D., C. Chauvin, R. P. Wilson, Y, Le Maho, and S. Wanless. 2005. Unusual feather structure allows partial plumage wettability in diving Great Cormorants Phalacrocorax carbo. Journal of Avian Biology 36: 57-63.
Griffin, T.M. and R. Kram. 2000. Penguin waddling is not wasteful. Nature 408:929.
Hedrick, T. L., J. R. Usherwood and A. A. Biewener. 2004. Wing inertia and whole-body acceleration: an analysis of instantaneous aerodynamic force production in cockatiels (Nymphicus hollandicus) flying across a range of speeds. Journal of Experimental Biology 207: 1689-1702.
Johanssen, L. C. and U. M. L. Norberg. 2001. Lift-based paddling in diving grebe. Journal of Experimental Biology 204:1687-1696.
Kvist, A., A. Lindstrom, M. Green, T. Piersma, & G. H. Visser. 2001. Carrying large fuel loads during sustained bird flight is cheaper than expected. Nature 413: 730 – 732.
Louw, G. J. 1992. Functional anatomy of the penguin flipper. Journal of the South African Veterinary Association 63: 113-120.
Lovvorn, J. R. 2001. Upstroke thrust, drag effects, and stroke-glide cycles in wing-propelled swimming by birds. American Zoologist 41: 154-165.
Mangold, O. 1946. Die nase der segelnden Vo¨gel ein Organ des Stro¨mungssinnes? Naturwissenschaften 33:19-23.
Norberg, R. A. 1981. Why foraging birds in trees should climb and hop upwards rather than downwards. Ibis 123: 281-288.
Nyakatura, J. A., E. Andrada, N. Grimm, H. Weise, and M. S. Fischer. 2012. Kinematics and center of mass mechanics during terrestrial locomotion in Northern Lapwings (Vanellus vanellus, Charadriiformes). Journal of Experimental Zoology 9999A:1–15.
Ortega-Jimenez, V. M., and R. Dudley. 2012. Flying in the rain: hovering performance of Anna’s Hummingbirds under varied precipitation. Proceedings of the Royal Society B, online early.
Pennycuick, C. J. 2002. Gust soaring as a basis for the flight of petrels and albatrosses (Procellariiformes). Avian Science 2: 1-12.
Pennycuick, C. J. 2008. Information systems for flying animals. In: Theoretical Ecology Series, vol. 5. Modelling the flying bird (C. J. Pennycuick, ed.), pp. 305-331. Elsevier Inc., Amsterdam, The Netherlands.
Sachs, G. 2005. Minimum shear wind strength required for dynamic soaring of albatrosses. Ibis 147: 1 – 10.
Tobalske, B.W. and K.P. Dial. 1994. Neuromuscular control and kinematics of intermittent flight in Budgerigars (Melopsittacus undulatus). Journal of Experimental Biology 187:1-18.
Tobalske, B. W., N. E. Olson, and K. P. Dial. 1997. Flight style of the Black-billed Magpie: variation in wing kinematics, neuromuscular control, and muscle composition. Journal of Experimental Zoology 279: 313-329.
Tobalske, B. W., W. L. Peacock, and K. P. Dial. 1999. Kinematics of flap-bounding flight in the Zebra Finch over a wide range of speeds. Journal of Experimental Biology 202: 1725-1739.
Usherwood, J. R., M. Stavrou, J. C. Lowe, K. Roskilly, and A. M. Wilson. 2011. Flying in a flock comes at a cost in pigeons. Nature 474: 494-497.
Videler, J. J. 2005. Avian flight. Oxford University Press, Oxford, UK.
Warrick, D.R., B. W. Tobalske, and D. R. Powers. 2005. Aerodynamics of the hovering hummingbird. Nature 435: 1094-1097.
Weimerskirch, H., T. Guionnet, J. Martin, S. A. Shaffer, and D. P. Costa. 2000. Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proceeding of the Royal Society of London B 267: 1869-1874.
Weimerskirch, H., J. Martin, Y. Clerquin, P. Alexandre, and S. Jiraskova. 2001. Energy saving in flight formation. Nature 413: 697 – 698.
Useful links:
The intermittent flight of Zebra Finches: Unfixed gears and body lift
“Underwater Flight” of the Penguin
Back to BIO 554/754 syllabus














 viewing window, and the other above the pool pointing straight down. This way, he could plot the bird’s movement in three dimensions and calculate diving parameters such as dive speed and angle of descent. Bridge (2004) found that instead of improving the bird’s diving performance, wing molt had an unexpectedly adverse effect. During molt, the birds dived a shorter distance with each flap of the wings, and energy output from the wing movement, as measured by work per flap, was also reduced, especially when both primary and secondary feathers were missing.
viewing window, and the other above the pool pointing straight down. This way, he could plot the bird’s movement in three dimensions and calculate diving parameters such as dive speed and angle of descent. Bridge (2004) found that instead of improving the bird’s diving performance, wing molt had an unexpectedly adverse effect. During molt, the birds dived a shorter distance with each flap of the wings, and energy output from the wing movement, as measured by work per flap, was also reduced, especially when both primary and secondary feathers were missing.
 Follow
Follow